Figures & data
Figure 1 Fingolimod first-dose observation experience and treatment satisfaction survey flow diagram.
Abbreviations: TSQM, Treatment Satisfaction Questionnaire for Medication; DMT, disease-modifying therapy; MS, multiple sclerosis; n, number.
Figure 2 Study accrual and attrition.
Abbreviations: N, number; MS, multiple sclerosis.
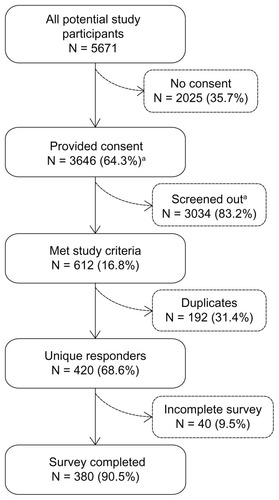
Table 1 Sociodemographic and clinical characteristics
Table 2 Reasons for starting or switching to fingolimod
Figure 3 First dose experience.
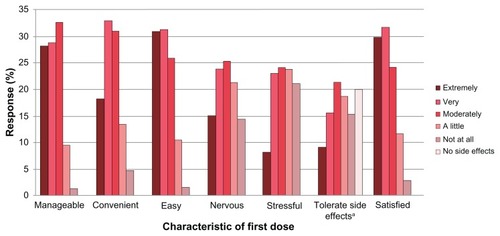
Figure 4 TSQM scale scores, overall and by treatment experience.
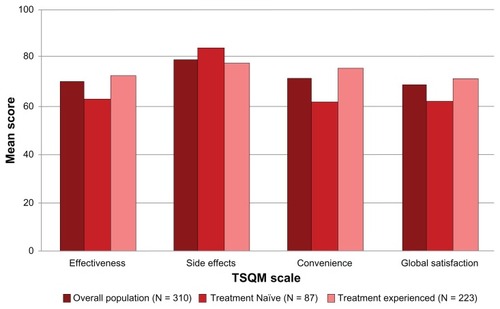
Table S1 Survey questions about first-dose experience