Figures & data
Figure 1 Relationship between the severity of airflow limitation and breathlessness as assessed by the mMRC questionnaire (panel A), exercise capacity as assessed by the 6MWD (panel B), reported exacerbations in the year before inclusion in the study (panel C), and health status as assessed by the SGRQ-C (panel D).
Abbreviations: mMRC, modified Medical Research Council; SGRQ-C, St George’s Respiratory Questionnaire – COPD specific; 6MWD, 6-minute walking distance.
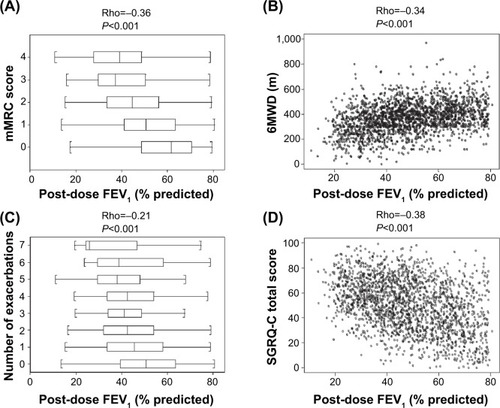
Table 1 Inhaled drugs for asthma and COPD
Table 2 Principal patient-reported scales and questionnaires for COPD
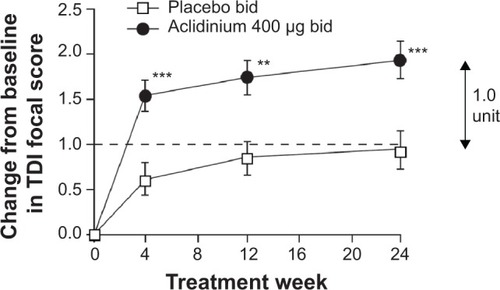
Figure 2 Change from baseline in the TDI focal score over 24 weeks.
Abbreviations: TDI, Transition Dyspnea Index; SGRQ, St George’s Respiratory Questionnaire; bid, twice daily.
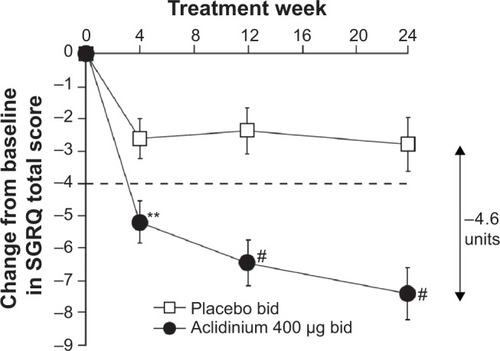
Table 3 Summary of efficacy outcomes of aclidinium bromide 400 μg vs placebo from the two main pivotal studies
Table 4 Adverse effects of aclidinium vs placebo in the ATTAIN and ACCORD COPD I studies
Figure 3 Change from baseline in the SGRQ total score over 24 weeks.
Abbreviations: SGRQ, St George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; bid, twice daily.