Figures & data
Figure 1 Study observation period for inception cohort.
Notes: Index date is the first prescription date in the index period. If patients are eligible multiple times for study inclusion during the observation period, only the first qualified medication episode is included.
Abbreviation: T2DM, type 2 diabetes mellitus.
Abbreviation: T2DM, type 2 diabetes mellitus.
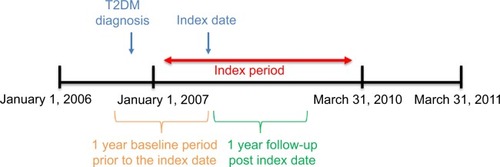
Figure 2 Adherence by concomitant medications and age groups.
Notes: *P<0.0001 versus reference group; †reference group for concomitant medications is ≥3 medications; ‡reference group for age is ≥65 years.
Abbreviation: PDC, proportion of days covered.
Abbreviation: PDC, proportion of days covered.
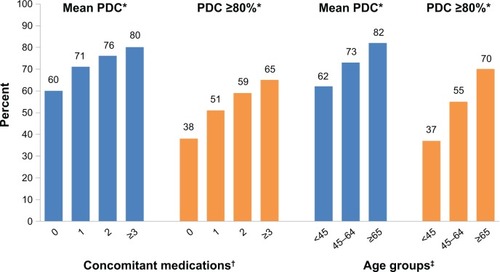
Figure 3 Adherence by age group and sex.
Note: *P<0.0001 versus reference group (≥65 years).
Abbreviation: PDC, proportion of days covered.
Abbreviation: PDC, proportion of days covered.
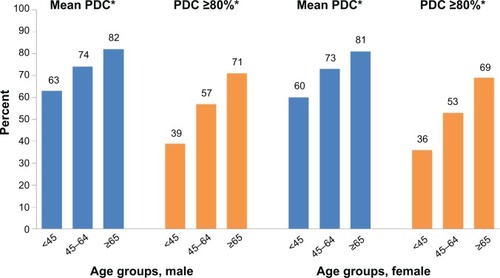
Table 1 Patient characteristics at baseline
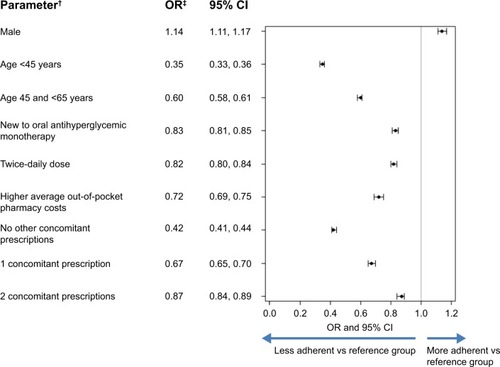
Figure 4 Logistic regression results for factors associated with adherence to oral antihyperglycemic monotherapy (PDC ≥80%)*.
Notes: *Adjusted for baseline characteristics and comorbid conditions; †reference categories: female, age ≥65 years, previously on oral antihyperglycemic monotherapy treatment, once-daily dose, and ≥3 concomitant prescriptions; ‡an OR >1 indicates a positive association with adherence and an OR <1 indicates a negative association with adherence.
Abbreviations: PDC, proportion of days covered; OR, odds ratio; CI, confidence interval; vs, versus.
Abbreviations: PDC, proportion of days covered; OR, odds ratio; CI, confidence interval; vs, versus.