Figures & data
Figure 1 The BETACONNECT™ device.
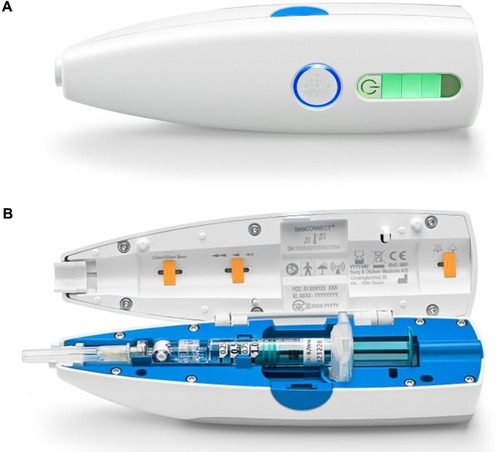
Table 1 Demographic characteristics of participants
Table 2 BETACONNECT™ – specific characteristics of participants
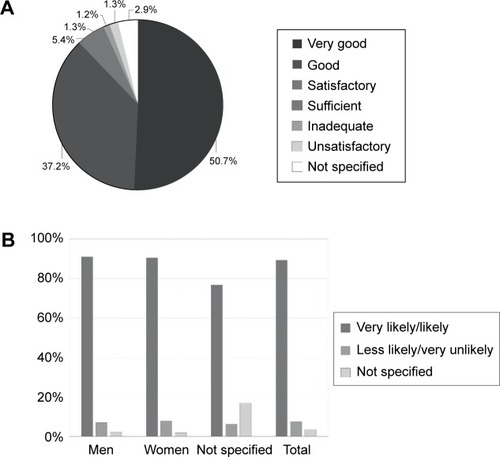
Figure 2 Rating of the support provided by the BETACONNECT™ device in interferon beta-1b therapy.
Abbreviation: NS, not specified.
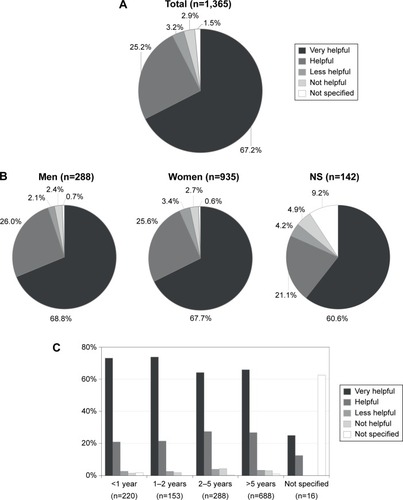
Figure 3 Rating of the BETACONNECT™ function and features in patient support (n=1,365).
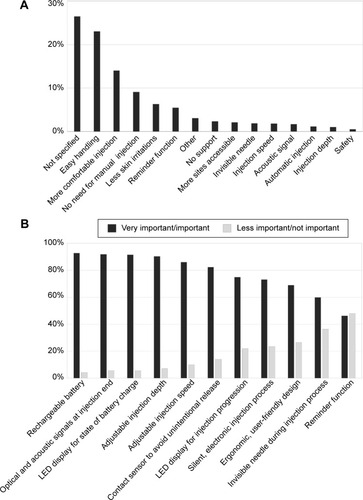
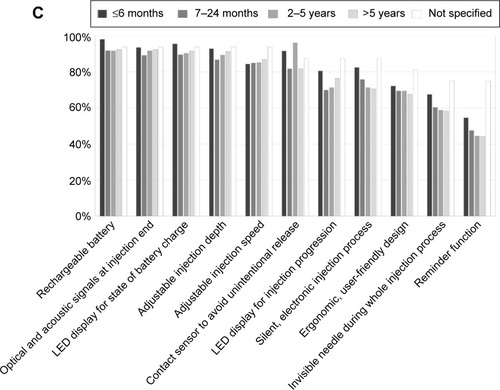
Figure 4 Evaluation of the BETACONNECT™.