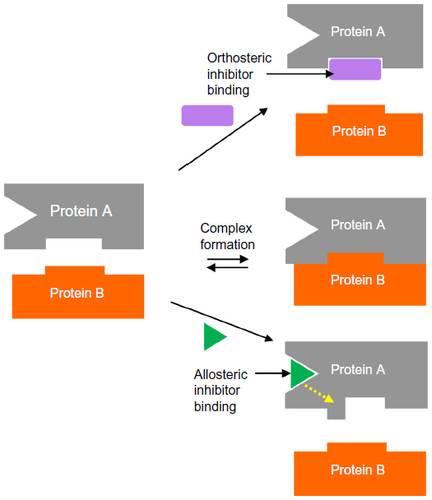
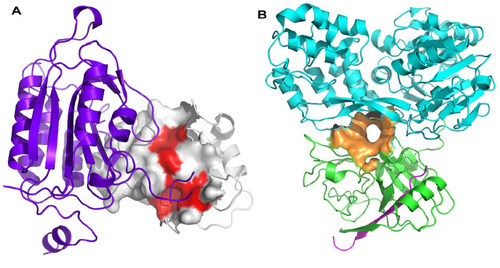
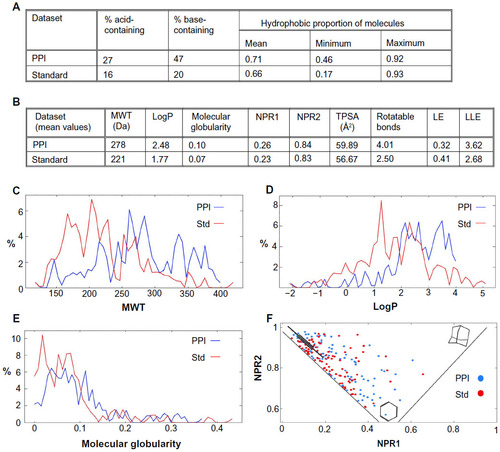
Figures & data
Table 1 Major screening and validation techniques used to detect PPI fragment hits
Table 2 Initial fragment hits and current leads for protein–protein interaction case studies