Figures & data
Figure 1 Novel “holistic” approach to ALS therapy. Clinical overlaps between ALS and other neurodegenerative diseases could unravel common molecular/pathological mechanisms (A). Moreover, new insights on causative genetic mutations (B) and the development of novel animal models (C) widen our knowledge of the possible therapeutic targets in the pathological pathways. In the meantime, recent iPS technology (D) provides patient-derived specimens as disease modeling and cell assays to dissect pathological mechanisms and specific cell contribution (E). The development of SC-based therapies is also directly exploitable for new drug screening (F and G). The discovery of the importance of epigenetic regulation in the pathological processes is paralleled by a relevant role in SC biology. Any alteration in this complex network could alter SC dynamic cross talk to the diseased surroundings, thus precluding possible therapeutic effects (H–J). The complex nature requires a multifaceted strategy, able to efficiently contrast widespread degeneration in all tissue districts (K), which should be carefully evaluated in accurate preclinical studies (L). Efficient therapeutic treatments are required both to replace MNs (M) and provide an healthy environment for them (N), capable also of enhancing endogenous repair (O). These laboratory studies will lead to successful clinical trials (P), based on novel surgical techniques (Q), able to slow the disease progression. Consensus international guidelines for drug/SC trials will guarantee the conscientious translation of basic SC research into appropriate treatment applications for patients aiming to create optimized efficient protocols able to slow down (neuro)degeneration (R).
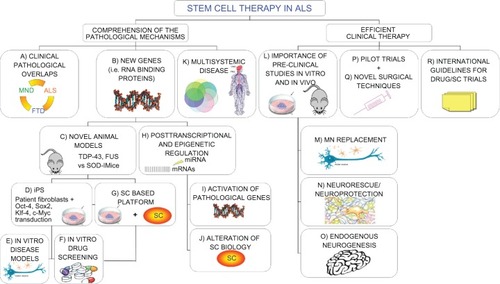
Table 1 Recent clinical trials with SCs in ALS patients (in chronological order)