Figures & data
Figure 1 Registry study design.
Abbreviation: DCP, data collection period.
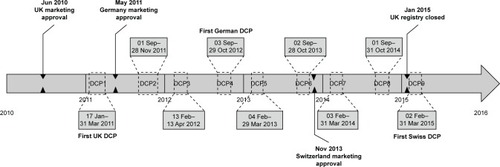
Table 1 Patient demographics
Figure 2 Patient duration of exposure to THC:CBD.
Abbreviations: CBD, cannabidiol; d, days; m, months; THC, Δ9-tetrahydrocannabinol; y, years.
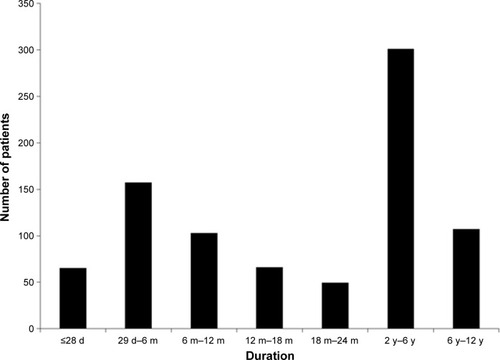
Table 2 Incidence rates for special interest events
Table 3 Most commonly reported AEs
Table 4 AEs presented by incidence and time to first onset category
Table 5 Most commonly reported SAEs
