Figures & data
Figure 1 Stepwise approach for the treatment of asthma in (A) patients aged 6 years and over and (B) children under 6 years of age, as recommended in the GINA Report.
Abbreviations: FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; HDM, house dust mite; ICS, inhaled corticosteroids; IgE, immunoglobulin E; Inc., increase; intermitt, intermittent; LABA, long-acting β2-agonist; LTRA, leukotriene receptor antagonist; med, medium dose; OCS, oral corticosteroids; SABA, short-acting β2-agonist; SLIT, sublingual immunotherapy; theoph, theophylline.
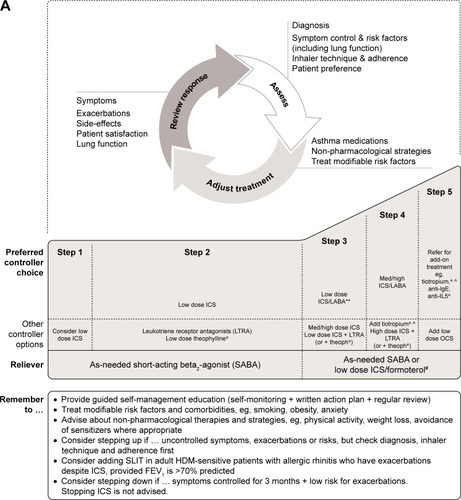
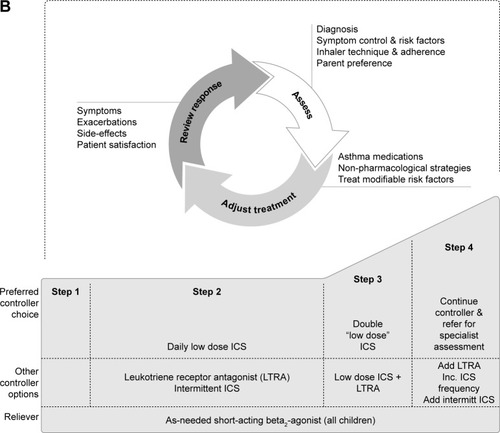
Table 1 Key results of Phase III studies with 2.5 and 5 µg tiotropium in children and adolescents with asthma
Table 2 Pharmacokinetics of tiotropium in asthma patients across age groups
Table 3 Asthma clinical trials in adults with LAMAs other than tiotropium
Table 4 Traditional and alternative clinical trial endpoints
