Figures & data
Figure 1 Changes in estimated proportion of women aged 45–69 years using menopausal hormone therapy in 17 European countries from 2002 to 2010 (A). Changes in estimated proportion of women aged ≥40 years reporting current use of oral postmenopausal hormones from 1999 to 2010 in the USA (B).
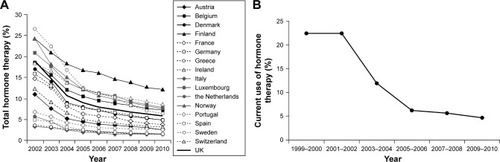
Figure 2 Incidence of breast pain during treatment with transdermal estradiol/oral micronized progesterone, oral CE/oral micronized progesterone, or placebo in women with and without baseline pain in KEEPS.
Abbreviations: CE, conjugated estrogens; KEEPS, Kronos Early Estrogen Prevention Study.
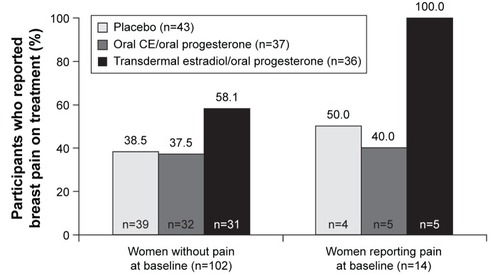
Table 1 Effects of CE/BZA on BMD at month 12 in SMART-1,Table Footnote& SMART-4,Table Footnote# and SMART-5Table Footnote$ (data on file)
Figure 3 Percentage of women reporting ≥1 day of breast tenderness in daily diaries during 4-week intervals through 12 weeks in SMART-5.
Abbreviations: SMART, Selective estrogens, Menopause, And Response to Therapy; CE, conjugated estrogens; MPA, medroxyprogesterone acetate; BZA, bazedoxifene.
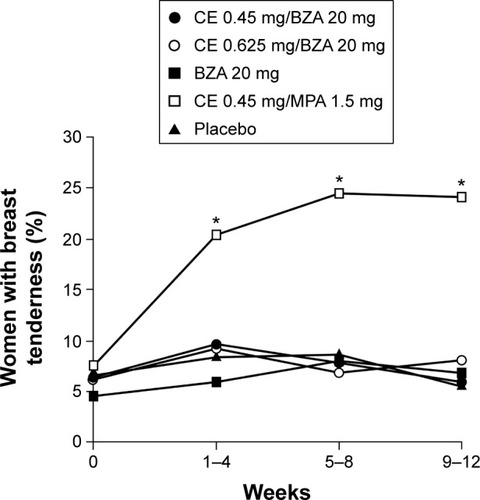
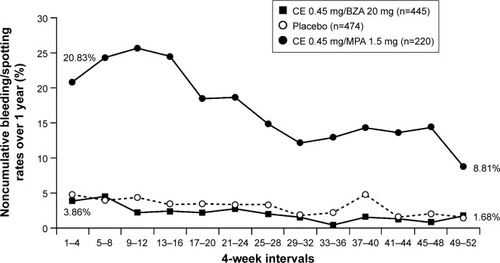
Figure 4 Bleeding/spotting rates over 1 year of treatment with CE/BZA compared to CE/MPA in SMART-5.Citation104
Abbreviations: CE, conjugated estrogens; BZA, bazedoxifene; MPA, medroxyprogesterone acetate; SMART, Selective estrogens, Menopause, And Response to Therapy.