Figures & data
Figure 1 HeFH and atherosclerotic CVD in 1090 patients with LDLC ≥70 mg/dL after ≥2 months maximal-tolerated cholesterol-lowering therapy.
Abbreviations: CVD, cardiovascular disease; HeFH, heterozygous familial hypercholesterolemia; LDLC, low-density lipoprotein cholesterol; MTDLLT, maximal-tolerated dose of standard-of-care LDL cholesterol-lowering therapy; PCSK9, proprotein convertase subtilisin/kexin type 9.
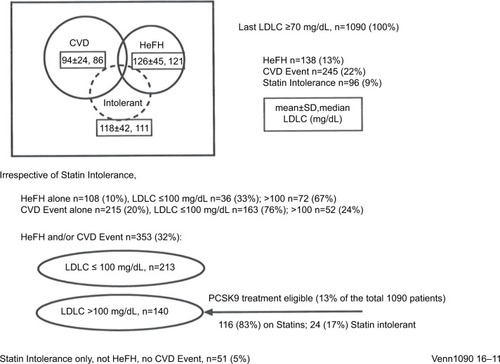
Table 1 LDLC in 353 patients with HeFH and/or CVD at entry and after 2 months on maximal-tolerated LDLC-lowering therapy
