Figures & data
Table 1 Characteristics and demographics of patients treated with raltegravir and a control population naïve to HAART
Table 2 Characteristics of HAART regimens used prior to first raltegravir regimen
Table 3 Antiretroviral drugs in first raltegravir regimen
Figure 1 Fraction of viral load (VL) tests below 51 copies/mL in 12 weeks intervals for the raltegravir cohort (full line) and the control cohort (broken line). Week 0 is start of raltegravir or HAART (index date).
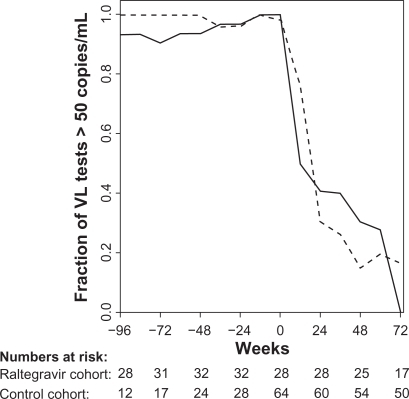
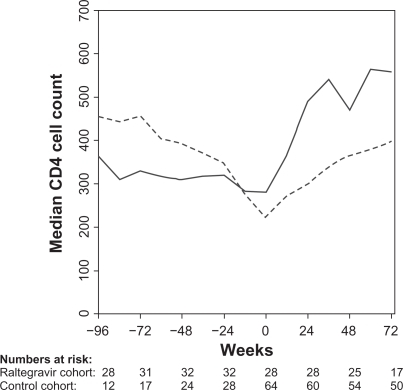
Figure 2 Median CD4 cell count at 12-week intervals for the raltegravir cohort (full line) and the control cohort (broken line). Week 0 is start of raltegravir or HAART (index date).