Figures & data
Figure 1 Remission is the key stepping stone between response to an acute episode and achieving full recovery (After CitationKupfer 1991).
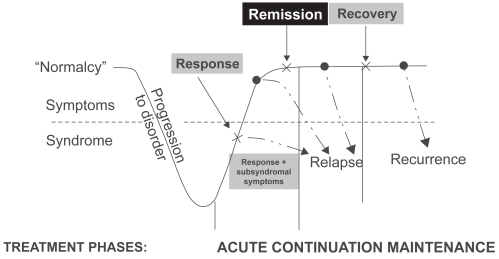
Figure 2 Remission at entry into follow-up was associated with lower relapse rates than response without remission in STAR*D study (12-month follow-up period) (After CitationRush et al 2006).
Relapse = QIDS-SR score ≥ 11.
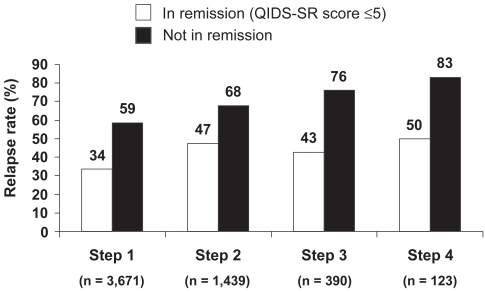
Figure 3 Meta-analysis of studies of atypical antipsychotic augmentation of antidepressants. CitationPapakostas GI, Shelton RC, Smith J, et al 2007a. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry, 68:826–31. Copyright © 2007 Physicians Postgraduate Press. Reprinted with permission.
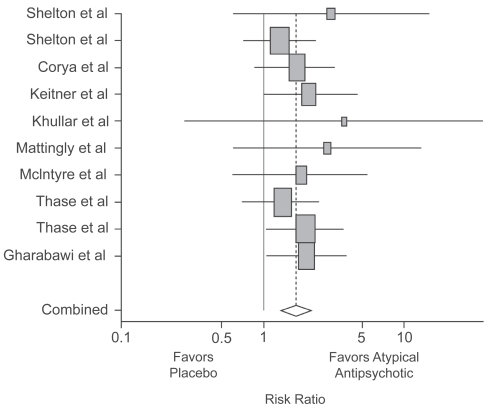
Table 1 Overview of clinical data for aripiprazole in major depressive disorder
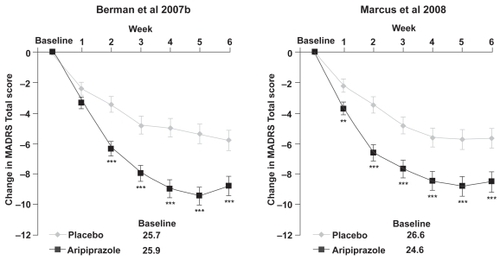
Figure 4 Change from baseline in MADRS Total score (last observation carried forward) in the two randomized, double-blind, placebo-controlled studies of adjunctive aripiprazole.
**p < 0.01 vs placebo.
***p < 0.001 vs placebo; MADRS Total score is rated from 0 to 60, where a negative change indicates improvement.