Figures & data
Table 1 Overview of randomized placebo-controlled double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization
Figure 1 Trial design used in a pregabalin randomized flexible-dose (150–600 mg/day) versus fixed-dose (600 mg/day) double-blind adjunctive-therapy trial in patients with refractory partial-onset seizures, with or without secondary generalization. Adapted from CitationElger et al 2005.
Figure 2 Seizure reduction in short-term fixed-dose pregabalin adjunctive therapy studies. Dose response relationship for seizure reduction (shown as response ratio [RRatio] on right y axis and percent change from baseline as calculated from RRatio on left y-axis) is shown for each of the three short-term fixed-dose pregabalin studies (French et al 2000; CitationArroyo et al 2004; CitationBeydoun et al 2005). P values shown represent a significant difference from placebo in the same study. Adapted from CitationBrodie et al 2004.
![Figure 2 Seizure reduction in short-term fixed-dose pregabalin adjunctive therapy studies. Dose response relationship for seizure reduction (shown as response ratio [RRatio] on right y axis and percent change from baseline as calculated from RRatio on left y-axis) is shown for each of the three short-term fixed-dose pregabalin studies (French et al 2000; CitationArroyo et al 2004; CitationBeydoun et al 2005). P values shown represent a significant difference from placebo in the same study. Adapted from CitationBrodie et al 2004.](/cms/asset/2b406f89-2795-4e4b-a811-ac8a23c539f8/dndt_a_4716_f0002_b.jpg)
Figure 3 Responder rates (proportion of patients with ≥50% reduction in seizure frequency compared with baseline) in each of four randomized adjunctive-therapy double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization. The first three trials (CitationFrench et al 2003; CitationArroyo et al 2004; CitationBeydoun et al 2005) included fixed-dose pregabalin treatment groups whilst the fourth trial (CitationElger et al 2005) included both flexible-dose (150–600 mg/day) and fixed-dose (600 mg/day) groups. *Significantly different from placebo in the same study.
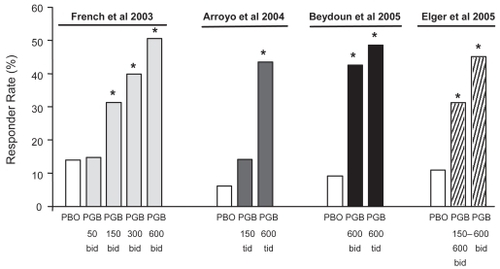
Table 2 Seizure freedom rates in patients treated with long-term adjunctive-therapy pregabalin
Table 3 Most common adverse events reported in randomized placebo-controlled double-blind trials of pregabalin in patients with refractory partial-onset seizures, with or without secondary generalization
Figure 4 Kaplan-Meier analysis of time to discontinuation due to adverse events with flexible (150–600 mg/day) versus fixed (600 mg/day) pregabalin dosing. Patients in the fixed-dose group discontinued from the study due to adverse events earlier than those in the flexible-dose group. Adapted from CitationElger et al 2005.
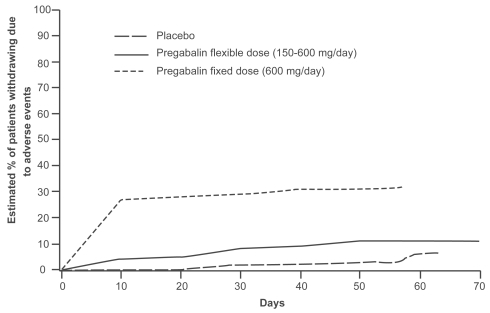
Table 4 Most common adverse events (reported by ≥10% of all patients) among 1480 patients treated with long-term adjunctive-therapy pregabalin based on pooled data from 4 open-label studies