Figures & data
Figure 1 Flow of patients from the double-blind phase through the open-label phase of the trial.
Abbreviation: OROS, osmotic release oral system.
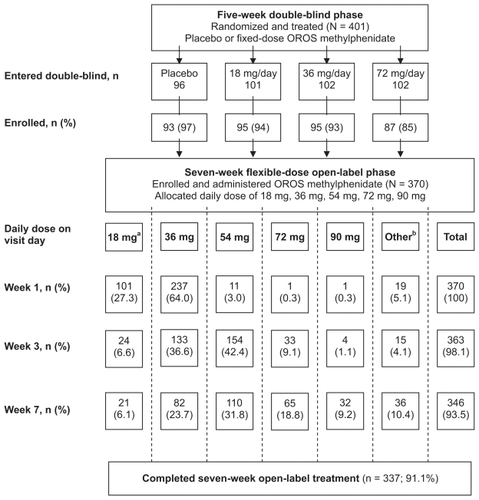
Table 1 Study medication administered during the open-label phase
Table 2 Adverse events during the open-label phase
Table 3 Percent of patients who reported an adverse event by daily dose and treatment period
Table 4 Adverse events by daily dose at final treatment visit
Table 5 Patients who met clinically relevant criteria for cardiovascular-related measurements by daily dose at final treatment visit