Figures & data
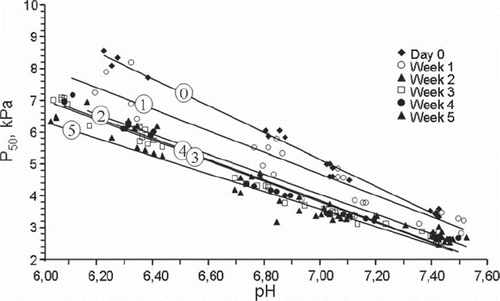
Figure 1. Changes in P50 values as a function of storage time measured at the four different pH values shown in the figure. All data from 0–17 days of storage represent blood from the same EC bags. The number of EC bags examined after each storage period is shown in italics above the x-axis. Data for all storage periods longer than 2 days were significantly different from day 0. The p values shown as numbers in the figure refer to a difference from the preceding P50 level by a paired T-test including all pH levels. The asterisks denote a significant (*p < 0.05) difference from the preceding value when each individual pH level was analysed separately.
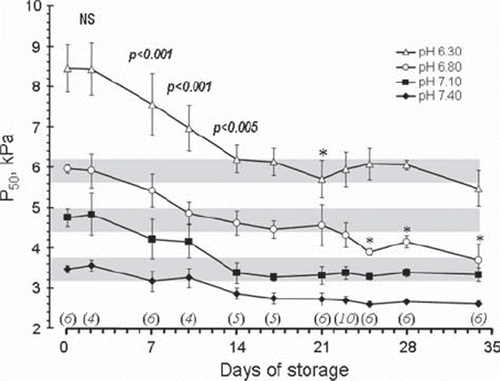
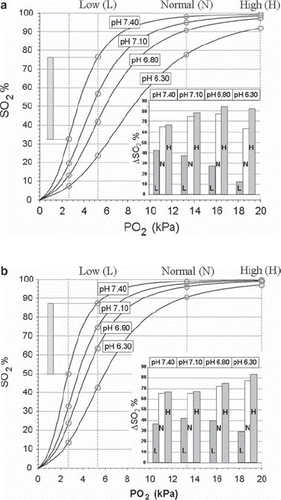
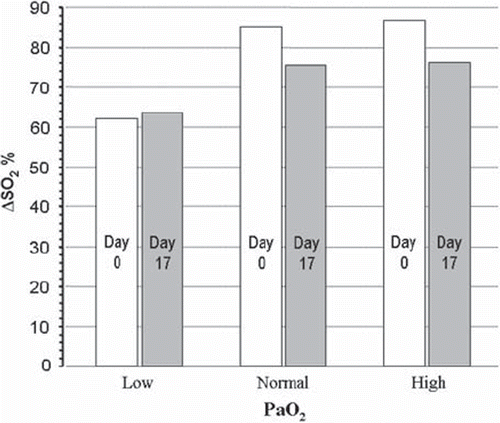
Figure 2. Actual HbO2 dissociation curves at 4 different pH values in blood from EC before storage (a) and after storage for 16–17 days (b). Vertical lines are drawn corresponding to PO2 values of 2.7 kPa (critical end-venous level), 5.3 kPa – Low (L), 13.3 kPa – Normal (N) and 20 kPa – High (H). The intersection between these lines and the HbO2 curves are marked with circles, the amount of consumable oxygen given as the ΔSO2 values depicted in the inserts was calculated as exemplified by the method shown for pH = 7.40 and PO2 = 5.3 kPa in both figures.