Figures & data
Figure 1. Morphology of cells isolated from human small bowel. (A) Five to six days after placing tissue pieces in culture plates, cells started to migrate out from (B) a preconfluent culture of EpCAM-positive cells. These cells grew and showed epithelioid morphology and could be maintained for 6–7 passages. (C) Confluent monolayer of EpCAM-positive cells showing cobble-shaped morphology typical of epithelial cells. (D) Transmission electron micrographs of cultured primary enterocytes showing presence of microvilli (arrows). Magnification 20×.
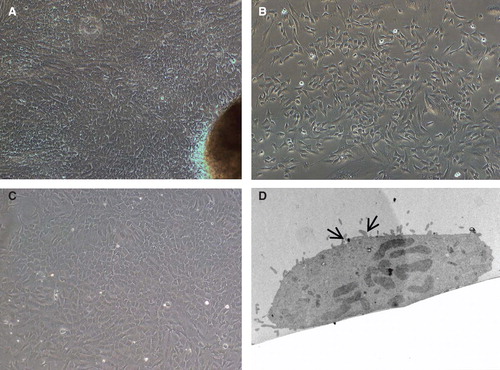
Table I. Markers expressed by human enterocytes isolated from small intestine.
Figure 2. Flow cytometric analysis of cultured human EpCAM-positive cells. (A) Flow cytometric results are presented in the form of histograms. Representative histograms for expression of TLRs in enterocytes are shown. Primary enterocytes did not express TLR-1, -2, -3, -4 and -9. They weakly expressed TLR-5, but demonstrated strong expression of TLR-6, -7, -8 and -10. The expression of TLRs was observed only in saponin-treated cells. (B) Isolated human enterocytes also stained positive for several immune recognition molecules such as CD44, CD86, ICAM-1 and CD40. Staining with only the secondary antibody served as negative control (grey filled histogram).
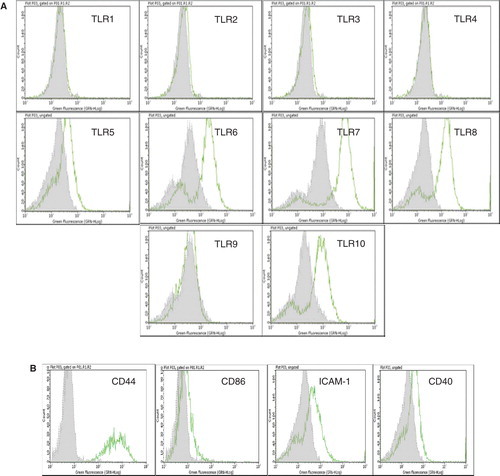
Figure 3. Immunocytochemical staining of isolated human enterocytes for epithelial markers. (A)Top panel: isolated and cultured human EpCAM cells stained positive for cytokeratins 18, 20 and on activation with IFN-γ also expressed HLA-DR. Bottom panel: enterocyte-specific markers such as maltase glucoamylase and sucrase isomaltase were also found to be expressed by the cells. Staining for mucins showed the presence of few secretory epithelial cells – goblet cells which stained pink/magenta, or blue indicating the presence of neutral and acidic mucins. (B) Immunofluorescence staining of human enterocytes with antibodies to Toll-like receptors (TLRs) showed that these cells expressed TLR-6, -7, -8 and 10, thus confirming our FACS results. Magnification 20×, neutral mucin and acidic mucin 60×.
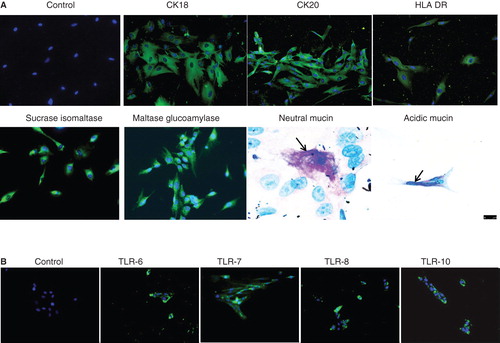
Figure 4. Immunofluorescence staining of normal human small bowel tissue sections with antibodies to various epithelial markers. Indirect immunofluorescence single staining was performed for detection of CK8, CK18, CK19 and CK20. Positive staining for CK8, CK18 and CK19 was observed along the whole epithelial layer of crypt–villus axis while CK20-positive staining was observed only at the differentiated villus cells. Indirect immunofluorescence double staining for EpCAM (green) and enterocyte markers, sucrase isomaltase and maltase glucoamylase (both red) in small intestine was performed. EpCAM-positive reaction is found on basolateral and basal side of epithelial cells while sucrase isomaltase and maltase glucoamylase-positive reaction is observed on luminal side only. Positive reaction for sucrase isomaltase is present in crypt as well as villus cells but for maltase glucoamylase it is observed only in villus cells. Magnification 40×.
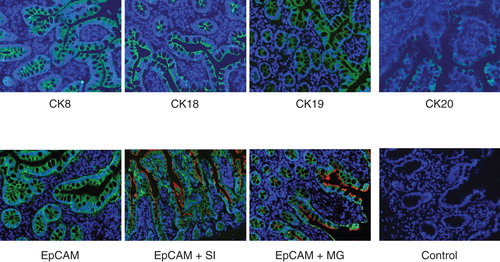
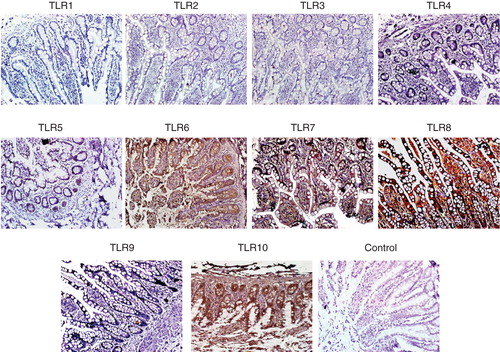
Figure 5. Immunohistochemical detection of TLR on paraffin sections of human small intestine. Small intestine biopsies from healthy individuals were stained for various Toll-like receptors (TLRs). No expression of TLR-1, -2, -3, -4 and -9 were found. However, the following TLRs were found to be expressed in the SI sections. TLR-5: weak positive staining is observed along the entire epithelial layer. TLR-6: epithelial layer showed moderate staining while intense reaction observed in the lamina propria cells and granules of paneth cells. TLR-7: both villus and crypt epithelial cells showed granular-positive reaction. TLR-8: diffuse positive staining of moderate intensity is observed along the whole epithelial layer. Many cells in the lamina propria also showed positive reaction. In two samples, intense positive reaction for TLR-8 is observed at the luminal side of epithelial layer; in these samples, few lamina propria cells showed positive reaction. TLR-10: intense positive reaction is observed in epithelial cells along the entire crypt–villus axis. Intense brown reaction was observed in the granules of paneth cells and also many lamina propria cells are intensely stained. Magnification 40×.