Figures & data
Figure 1. (A) Regulation mechanism of HSP. Environmental stresses (stressor) induce heat shock transcription factor (HSF), mainly HSF1. Under unstressed conditions, HSF1 binds to Hsp90 or Hsp70, and HSF1 is inactivated. Under stressed condition, Hsp90 or Hsp70 dissociates from HSF1 to bind to degenerated proteins for assistance of their folding. Free HSF1 can be activated followed by trimerisation and phosphorylation, and obtains ability to move into the nucleus to bind to HSE. (B) Hsp70 induction by drug (GGA). The heat shock transcription factor HSF1 is suppressed by binding of constitutively expressed Hsp70 (HSC70) through its C-domain in the cytosol under normal conditions. GGA binds to the C-domain of the Hsp70, resulting in dissociation of the Hsp70 from HSF1. Then HSF1 is activated and the trimers can be formed. The activated HSF1 moves into the nucleus from the cytosol and HSF1 binds to HSE.
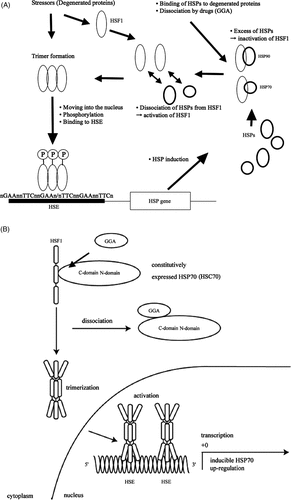
Table I. Possible mechanism for HSP induction in vivo-mode of action of stressors.
Table II. Induction and cytoprotective function of HSPs in upper gastrointestinal mucosa.
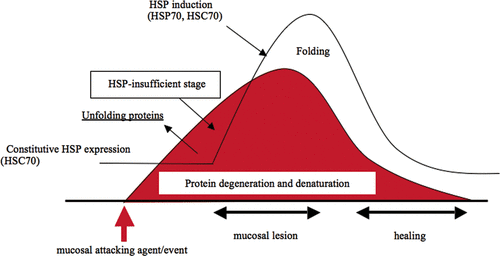
Figure 2. Gastric mucosal damage and protection by Hsp70 family. When gastric mucosa is exposed to attacking agents or events, degeneration or denaturation of cellular proteins is started. As an acute stress response, constitutively expressed cognate Hsp70 (HSC70) recognises and binds to denatured proteins to fold or normalise the structure. However, when attacking agents are strong enough to develop further cellular damage, HSP-insufficient stage may occur prior to ulcer formation.