Figures & data
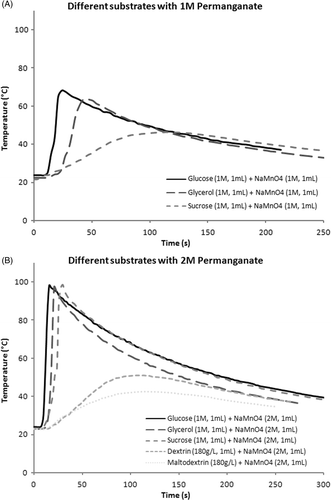
Figure 1. (A) Temperature profile of simultaneous injection of glycerol and permanganate under various conditions. (B) Temperature profile of simultaneous injection of glucose and permanganate under various conditions. (C) Temperature profile of simultaneous injection of sucrose and permanganate under various conditions. Note blunting of rise and reductions in peak temperatures compared to 1A and 1B.
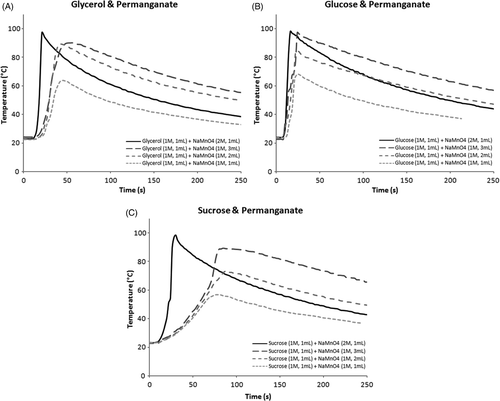
Table I. Temperature increases and peak temperatures with different stoichiometries using glycerol, glucose, and sucrose as substrates.
Figure 2. (A) Temperature data from simultaneous ex vivo injections of glucose and permanganate into muscle tissue using only 500 microlitres each. (B) Gross specimen bisected after simultaneous injection of glucose and permanganate into muscle tissue under conditions listed for . Staining from permanganate products is apparent. (C) Infrared pseudo color image from sample in , simultaneous injection of glucose and permanganate with warmed dime for a reference. Temperature range 19.8–58.2°C. Note relatively large area still off scale despite delay for tissue sectioning prior to imaging.
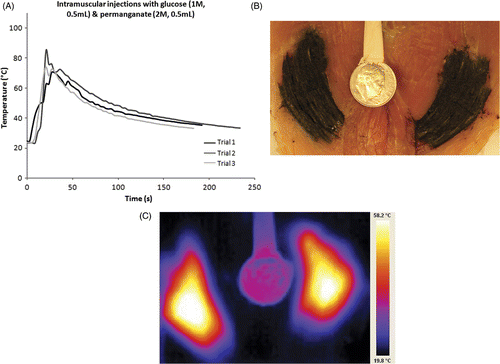
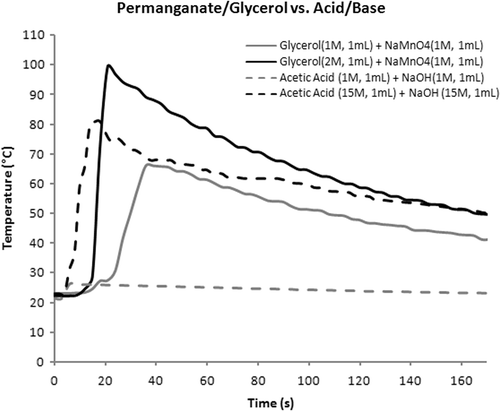
Figure 3. Comparison of redox and neutralization reactions. Note that the exotherm from 1 M acid and base is near baseline and peak temperature from 15 M solutions is intermediate between 1 and 2 M redox values.