Figures & data
Figure 1. Illustration of the change in the opacity of a BSA polyacrylamide hydrogel due to heat-induced denaturation of the embedded proteins. The opaque lesions were created by exposure to High Intensity Focused Ultrasound (HIFU) generated by the transducer shown in the background.
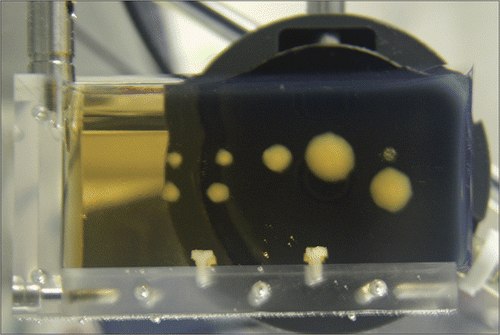
Figure 2. Optical transmittance of 3.5% (w/v) BSA solution exposed to various temperatures for 30 minutes. The data were obtained using a UV/VIS spectrometer set to 350 nm and with 100% transmittance corresponding to distilled water containing no BSA. The values plotted here are averaged over 3 separate runs of the experiment described in the text. Standard deviations are indicated with error bars.
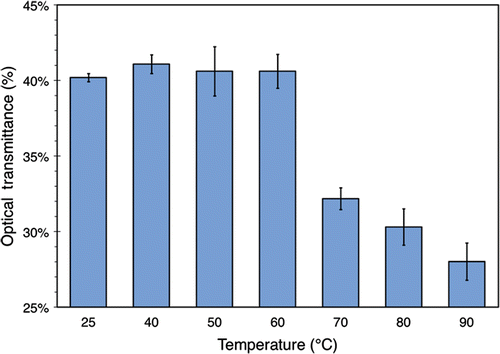
Figure 3. Minutes 6-12 of three SEC chromatograms for 3.5% (w/v) BSA solutions heated to 25°C, 70°C, and 90°C for 30 minutes. Absorbance was monitored at 280 nm. For this run of the experiment, the chromatograms at 40°C, 50°C, and 60°C (not shown) were found to be similar to the 25°C one. Absorbance at all other times was found to lie below the noise floor.
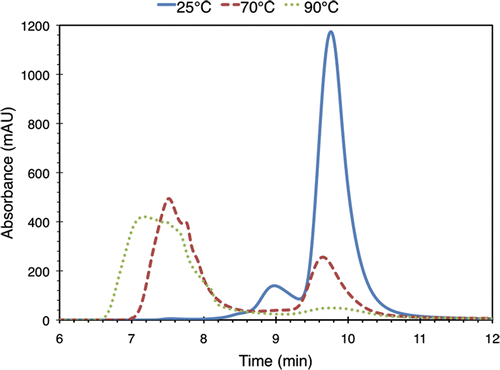
Figure 4. The relative amount of BSA monomers and aggregates in 3.5% (w/v) BSA solution exposed to various temperatures for 30 minutes. The data were obtained by performing peak identification and integration on the associated SEC chromatograms. The values plotted here are averaged over 3 separate runs of the experiment described in the text. Standard deviations are indicated with error bars.
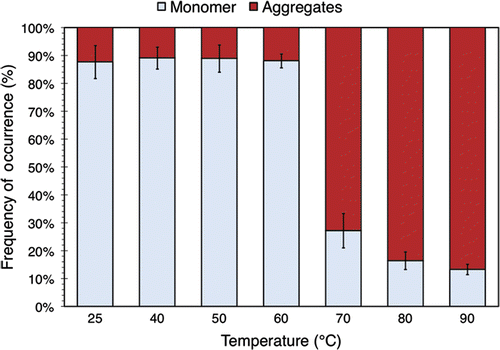
Figure 5. (a) FTIR spectra of 2% (w/v) BSA solutions heated to 25°C, 70°C, and 95°C for 30 minutes. For this run of the experiment, the spectra at 40°C and 60°C (not shown) were found to be similar to the 25°C one. (b) Second derivatives of the spectra. The zero-crossings of the second derivative curves correspond to inflection points in the original spectra, which are used in the peak fitting process as described in the text.
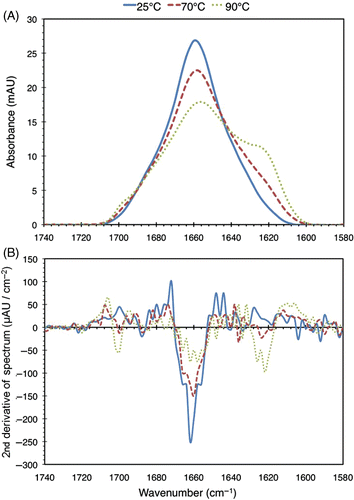
Figure 6. Relative secondary structure distribution of BSA in solutions with a concentration of 3.5% (w/v) exposed to elevated temperatures for 30 min. The data were obtained using a FTIR apparatus. The values plotted here are averaged over 3 separate runs of the experiment described in the text. Standard deviations of more than 0.5% are indicated with error bars.
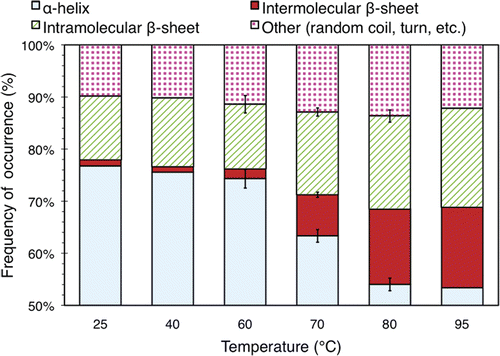
Figure 7. Survival of ZR75.1 human breast cancer cells after 1 min of heating, expressed as a percentage of a control sample that was not heated. Cell survival was measured using an MTS assay. The temperature indicated is that of the water bath used to heat the samples, although the internal temperature of the samples themselves had settled to the within 1°C of this value by the end of the heating process. The values plotted here are averaged over 3 samples from one run of the experiment described in the text. Standard deviations are indicated with error bars.
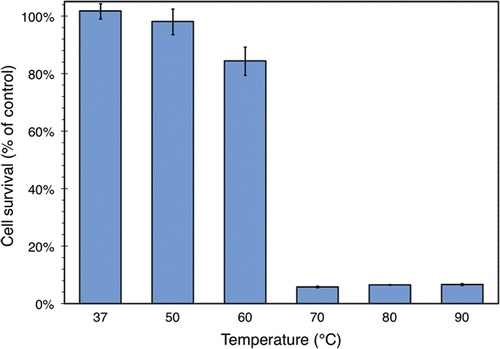
Figure 8. Normalized optical transmittance of a polyacrylamide hydrogel with embedded BSA after 1 min of heating. The experimental protocol and the normalization procedure are described in the text and in Citation[7]. The temperature indicated is that of the water bath used to heat the gel, although the internal temperature of the gel slices themselves had settled to the within 1°C of this value by the end of the heating process. The values plotted here are averaged over 3 separate experimental runs. Standard deviations are indicated with error bars.
![Figure 8. Normalized optical transmittance of a polyacrylamide hydrogel with embedded BSA after 1 min of heating. The experimental protocol and the normalization procedure are described in the text and in Citation[7]. The temperature indicated is that of the water bath used to heat the gel, although the internal temperature of the gel slices themselves had settled to the within 1°C of this value by the end of the heating process. The values plotted here are averaged over 3 separate experimental runs. Standard deviations are indicated with error bars.](/cms/asset/de48791f-d2ba-481f-a16a-c750cc78a524/ihyt_a_479451_f0008_b.gif)
