Figures & data
Figure 1. Experimental setup for in vivo MRI-controlled focused ultrasound hyperthermia. (a) Axial depiction of rabbit in lateral decubitus position. (b) Axial T2-weighted image along beam path, indicating the coronal plane used for MRI thermometry and the acoustic field path at the boundaries of the heating trajectory. (c) Coronal T2-weighted image taken through the plane indicated in (b), with eight control regions shown on the periphery of the 10mm diameter target region. (b) and (c) both have a 120 mm field of view.
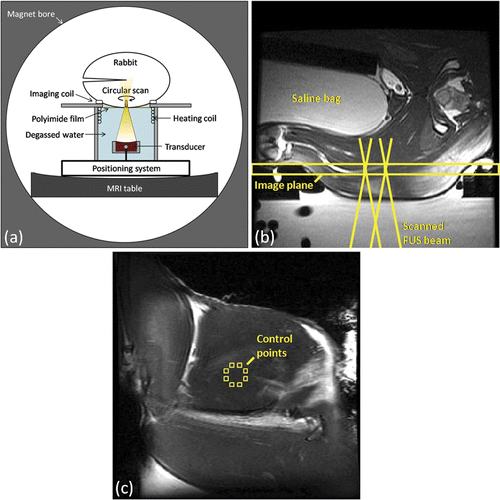
Figure 2. Simulations (a,c,e) and in vitro (b,d,f) testing of proportional-integral MRI temperature control for mechanically-scanned focused ultrasound hyperthermia. Mean, T90 and T10 temperatures in the 10 mm diameter circular region of interest for a target temperature elevation (Tgoal) of 10°C using controller gains KP = 4.5, KI = 0.03 and an exponential input function with time constant tau = 160 seconds, in simulation (a) and in vitro, heating a tissue-mimicking gel phantom (b). Temperature maps at 10 minutes after the start of heating, and thermal dose maps calculated from the same simulation (c,e) and in vitro (d,f) controlled heating data, assuming a target temperature of 43°C. 10 mm diameter circular scan trajectory outline in white.
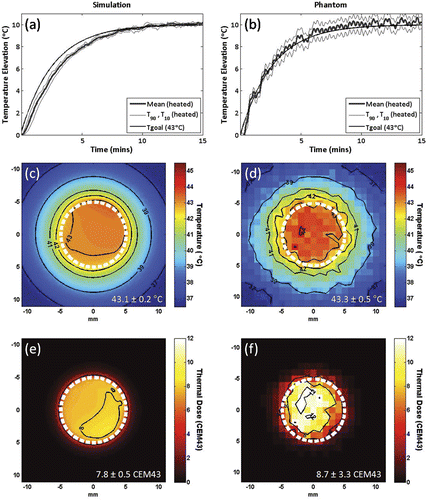
Figure 3. Evolution of temperature distribution during MRI-controlled focused ultrasound heating of a 10 mm diameter region. Control ROIs, circular unheated ROI, and temperatures greater than 37°C are overlaid on coronal FSPGR magnitude images after (a) 0, (b) 5, (c) 10, and (d) 20 minutes of heating.
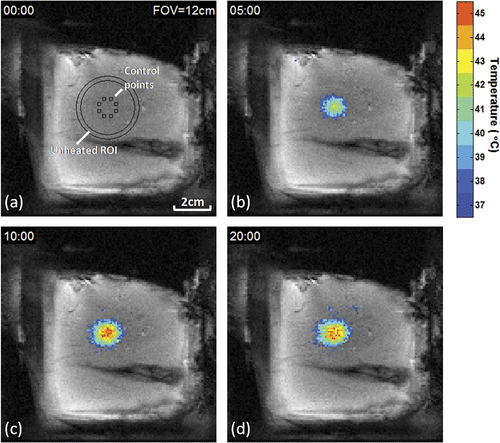
Figure 4. Example of temporal and spatial temperature control over a 10 mm diameter circular area using MRI-controlled focused ultrasound hyperthermia. (a) Mean, T90 and T10 temperatures in the circular region of interest. Lyso-thermosensitive liposomal doxorubicin (LTLD) infusion duration is shaded. At the end of heating, the transducer returned to the center position and reduced temperature noise was observed. (b) Time-averaged mean ± SD temperature versus radius. Unheated region of interest is shaded. (c) Thermal dose map centered on target region, circular scan trajectory outlined in white.
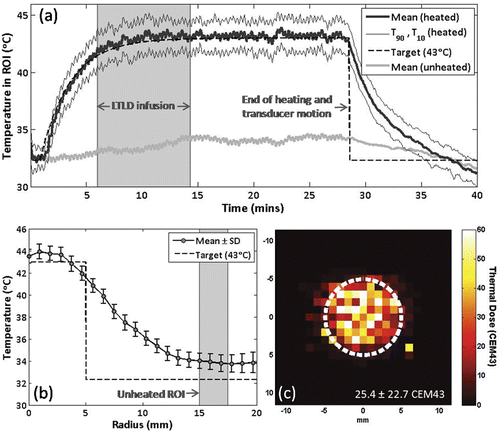
Figure 5. Tissue damage observed using T2-weighted and contrast-enhanced T1-weighted imaging following thermal coagulation in rabbit thigh. (a,b) T2-weighted axial (a) and coronal (b) images through the center of the heated region. (c,d) Corresponding contrast-enhanced T1-weighted axial (c) and coronal (d) images from the same experiment. 80 mm field of view, target regions (outlined) and thermal damage boundaries (arrows) shown.
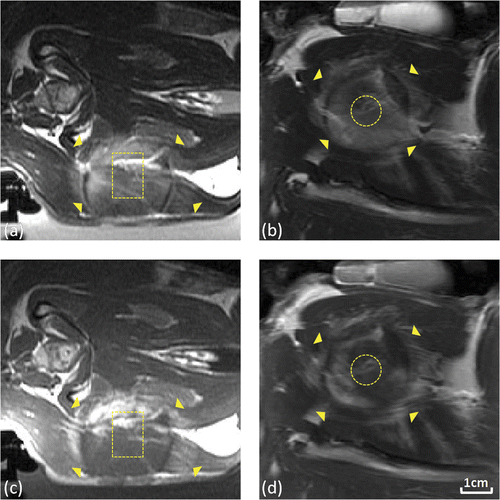
Table I. Summary of in vivo MRI-controlled FUS experiments for hyperthermia-mediated drug delivery.
Figure 6. Doxorubicin concentrations measured by fluorescence intensity in tissue samples harvested from heated and unheated regions of thigh muscle in rabbits receiving controlled hyperthermia (n = 6) or thermal coagulation (n = 4). Columns, mean; bars, SD. *P<0.05, Wilcoxon matched pairs signed rank test.
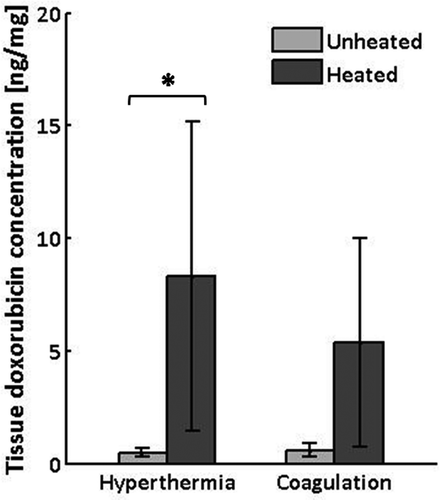
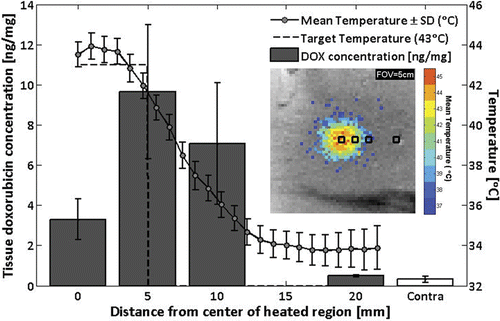
Figure 7. Doxorubicin concentrations measured by fluorescence intensity in tissue samples from rabbit #9, harvested at 0, 5, 10, and 20 mm away from the center of the heated region, as well as from the unheated contralateral thigh (mean ± SD). Overlay: time-averaged temperature profile. Inset: sample positions shown on image of mean temperatures greater than 37°C.