Figures & data
Figure 1. Release from iLTSL in HEPES buffer and human plasma. (a) Release of Dox as a function of temperature is shown as the sample is heated from 20 to 55°C at 1°C/min. Note that in this graph, the influence of temperature and time are coupled. (b) Dox release as a function of time at a constant temperature. Percent release is calculated by assuming 100% release with TritonÒ X-100 and 0% release at 25°C. (c) and (d) show the same data for release in human plasma.
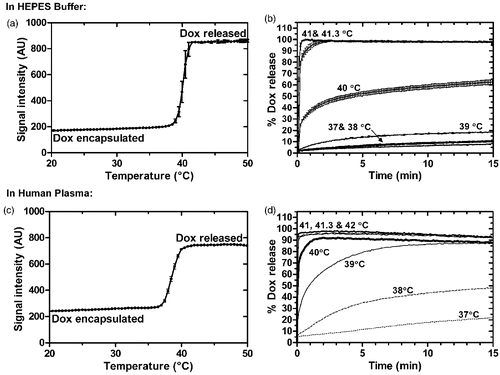
Table I. Summary of both kinetic and ThermoScan Dox release assays in HEPES buffer and human plasma.
Figure 2. Release of Dox and Gd-HP-DO3A from iLTSL. Release in HEPES: (a) Percent release vs. time and fitted curves for Dox (○--) and Gd-HP-DO3A ((•–) over 10 min. (b) The first minute of release. Percent release values were not significantly different between Dox and Gd-HP-DO3A over 10 minutes (p > 0.05, Dunn's multiple comparison). Release in plasma: (c) Percent release vs. time for Dox and Gd-HP-DO3A over 10 min. d) The first minute of release. Percent release values were not significantly different between Dox and Gd-HP-DO3A in either HEPES or plasma (p > 0.05, Dunn's multiple comparison). Each point represents the mean of 3 experiments ± SEM.
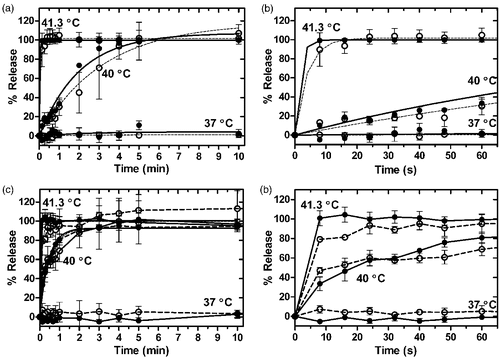
Figure 3. Stability of Gd-LTSL-DOX. a) Release of doxorubicin immediately (dashed line) and 7 days after synthesis (solid line) of Gd-LTSL-DOX at 37, 40 and 41°C in HEPES buffer. b) Point-by-point difference between release curves at 37, 40 and 41°C obtained 1 week apart (%release at day 0 – %release at day 7). Symbol size indicates temperature: 37°C is smallest (○--) and 41°C is largest (•–).
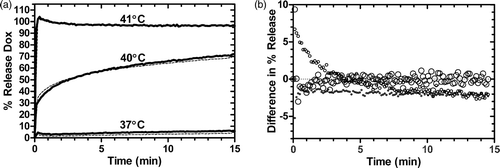
Figure 4. Relaxation rate (R1) versus concentration of Gd-HP-DO3A at 1.5T. Gd-LTSL-DOX solutions were heated in a water bath (55°C for 5 min) to release Gd-HP-DO3A and the drug. The resulting relaxivity (slope) values for heated and unheated iLTSL were 4.01 ± 0.01 and 1.95 ± 0.05 mM-1s-1, respectively, and were significantly different (p < 0.0001, F test). Relaxivity of Gd-HP-DO3A (4.05 ± 0.14 mM-1s-1) was not significantly different from that of heated iLTSL (p = 0.85, F test). R2 > 0.992 for all fitted data.
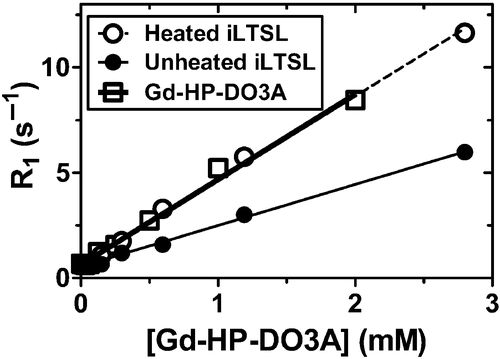
Table II. Summary of Gd-HP-DO3A and Dox release from Gd-LTSL-DOX in HEPES and Plasma.
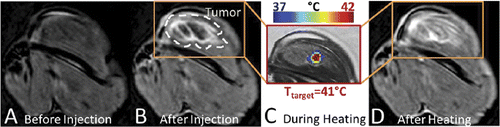
Figure 5. Location of release of iLTSL contents can be controlled and visualized with MR-HIFU. iLTSL suspended in 2% agar-silica demonstrated baseline intensity (a) until heated above the Tm of the liposome with MR-HIFU (b - d). The bright shapes result from different sizes and shapes of regions that were heated with MR-HIFU.

Figure 6. MR signal intensity during heating with MR-HIFU of a silica-agarose gel phantom containing iLTSL. a) MR signal intensity in heated, unheated, as well as preheated regions. b) Temperature in the same regions, as measured with MR thermometry. Signal intensity of the region where the MR-HIFU energy is focused increased most likely due to Gd-HP-DO3A release. The signal intensity in a region that has been previously heated did not change drastically, but remained high relative to unheated regions. An unheated region demonstrated stable and relatively low signal intensity. Regions of interest contained 20–45 voxels. Mean signal/temperature ± SEM is shown.
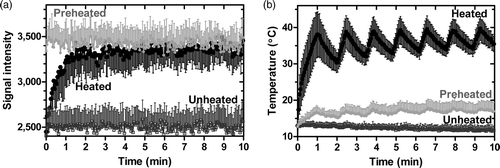
Figure 7. MR signal intensity before and after iLTSL injection and heating with MR-HIFU. Signal intensity a) before iLTSL injection and b) after iLTSL injection. c) Example of temperature map during heating, overlaid on signal intensity obtained with a treatment planning proton density weighted scan. d) Signal intensity after four 10-minute heating sessions. Note that (a), (b) and (d) depict T1-weighted images whereas (c) shows a proton density weighted image.