Figures & data
Figure 1. Stress induced activation of autophagy in aging. Proteotoxic stress or ROS or other stress in aging can activate FoxO and Nrf2 which in turn can activate autophagy by activating LC3I and sequestosome 1/p62 respectively. HSF1 is also activated in stress which may regulate transcription of Nrf2 and FoxO3 maintaining protein homeostasis in cells.
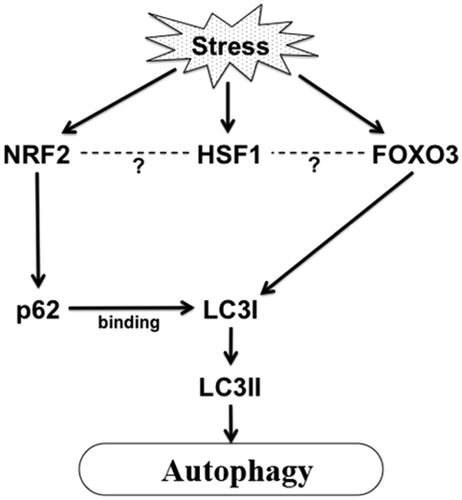
Figure 2. Chaperones and their transcription factor, HSF1 play significant role in multiple processes in cells under stress. HSF1 is activated under stress, which causes HSF1 to form a trimer. Active HSF1 then translocates to the nucleus and directly bind to the promoter of the HSP gene, thereby activating transcription. HSP can fold unfolded protein that accumulates in aging. Chaperones can protect cells from proteotoxicity, protein aggregation, apoptosis and accumulation of misfolded proteins.
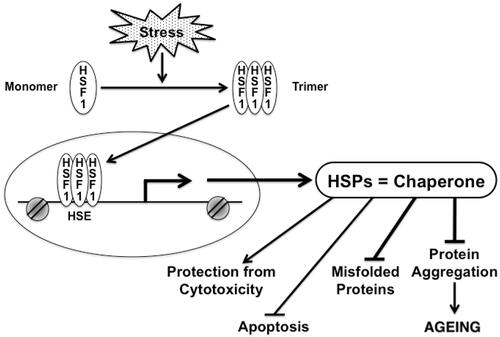
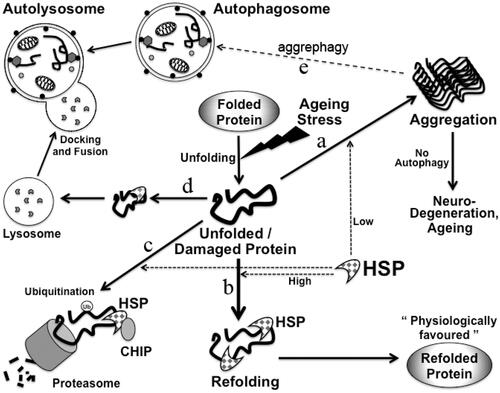
Figure 3. Maintenance of protein homeostasis by HSP in cells. Damaged or misfolded proteins accumulate in cells in aging. Protein homeostasis needs to be maintained in cells in aging to avoid aging-related diseases. Possible fates of misfolded proteins are shown: misfolded/unfolded proteins if not refolded can form aggregates (a), unfolded proteins can be refolded by HSP (b). These misfolded proteins can be degraded in proteasome through binding to Hsp70 and CHIP. CHIP leads to ubiquitinylation of these proteins (c). These misfolded proteins can also be transported to the lysosomes for degradation by CMA (d). Protein aggregates can also be degraded by aggrephagy (e).