Figures & data
Figure 1. Timeline delineating critical events during the case study. The vaccine was administered once a week for 44 weeks, from the first administration on 16 September 2011. Imiquimod was applied topically before and after the vaccine was given for the first 12 weeks of treatment. BCG was administered prior to the vaccine at week 30. Recurrent nodules were seen on radiographs/CT on 6 March 2012 – 6 months into treatment. The patient was euthanised on 30 July 2012, following development of plural effusion and a significant gastrointestinal haemorrhage.
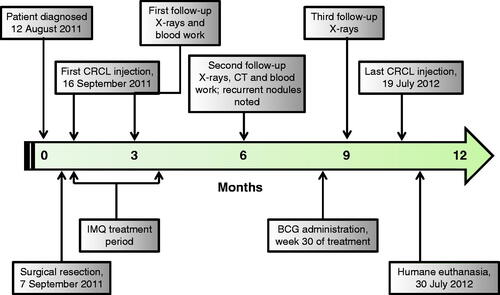
Figure 2. Imaging studies conducted (A) at diagnosis, (B) 3 months post-surgical, (C) 6 months post-surgical. CT studies were only completed at initial diagnosis and at the 6-month time point, when metastasis was suspected from radiographs. Thoracic radiographs were conducted at all time points.
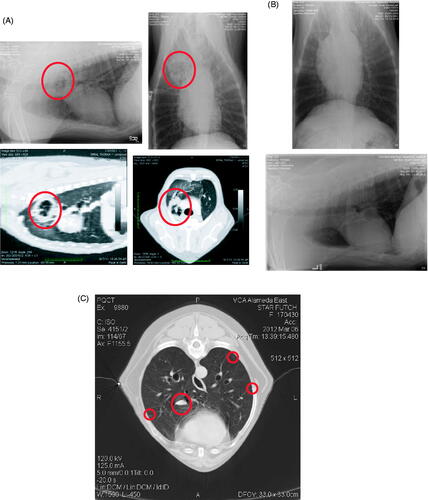
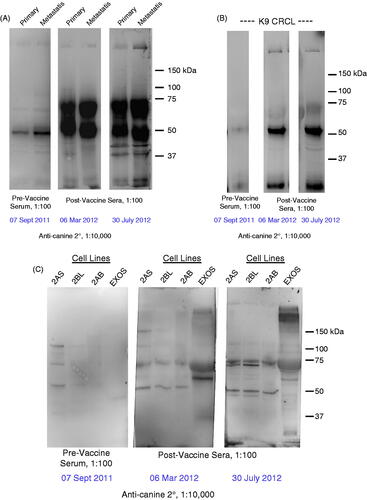
Figure 3. Western blot results using patient anti-sera to probe lysates prepared from (A) primary and metastatic tumours, (B) the CRCL preparation that was used as a vaccine, (C) cell lines generated from metastatic tumours and exosomes harvested from those lines. In each case, the sera are diluted 1:100, and consist of serum taken prior to receiving any vaccines (pre-vaccine serum), and sera taken ∼6 months post-vaccine initiation and ∼10 months post-vaccine initiation. Anti-canine IgG-HRP (1:10 000 dilution) was used as a secondary antibody, and blots were developed with ECL reagents. Molecular weight markers are shown on the right.