Figures & data
Table 1. Characteristics of available bladder hyperthermia thermometry systems.
Figure 1. Radiometric versus fibre-optic temperature. Bladder model: fluid is circulated from a temperature-controlled bath to a 30-mL Foley balloon located at 3.5 cm depth. The radiometer measures the ‘average’ bladder temperature, potentially missing extreme temperatures, whereas the fibre-optic probe measures at just a single point. Note the circa 5 min delay in the volumetric data from the radiometer versus the invasive fibre, located at the beginning of the bladder.
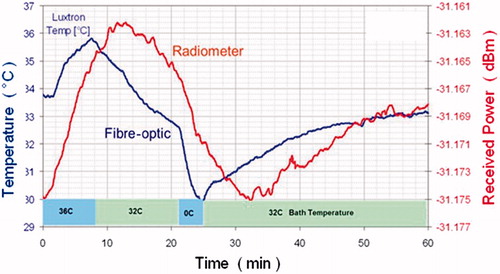
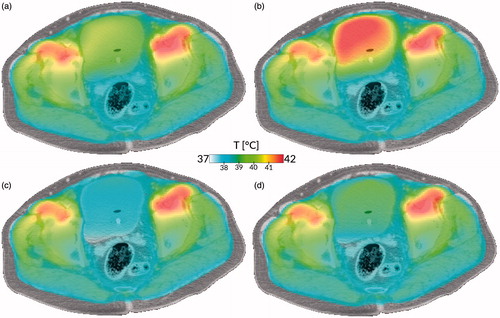
Figure 2. Impact of tissue properties and fluid modelling on treatment simulation. (a) Current state of the art: current tissue properties, no convection. (b) With updated dielectric properties, but no convection. (c) With convective modelling, but current tissue properties. (d) With updated dielectric properties and convective modelling. All cases show the simulation using the AMC hyperthermia treatment planning system of a bladder hyperthermia treatment using clinically applied equipment settings, after 15 min of heating. Case (a) shows a moderately heated bladder with a slightly heterogeneous temperature distribution, based on the currently used simulation settings treating the bladder as solid muscle. Because of the higher electric conductivity, more power is deposited inside the bladder, leading to a higher temperature for case (b). Cases (c) and (d) show more homogeneous but lower bladder temperatures because of the more effective method of heat conduction, resulting in faster removal of heat from the bladder to the surrounding musculature, even for the higher power uptake in case (d). The difference in dielectric properties (left versus right) is explained in the dielectric tissue properties section, the important effects of convection (top versus bottom) in the section on thermophysical bladder properties.