Figures & data
Figure 1. Adjusted* mean changes from baseline in % of withdrawals due to predefined asthma events± by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. ±Predefined criteria for an asthma event included: (1) decrease in am predose FEV1 ≥20% from randomization or a decrease to <45% (study I) or <40% (study II) of predicted normal, (2) ≥12 actuations of albuterol/day on ≥3 days within a 7-day period, (3) decrease in am PEF ≥20% from baseline on ≥3 days within a 7-day period, (4) ≥2 nights with an awakening due to asthma requiring rescue medication within any 7-day period and (5) clinical exacerbation requiring emergency treatment, hospitalization or use of an asthma medication not allowed by the protocol. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; LLN, lower limit of normal; PBO, placebo.
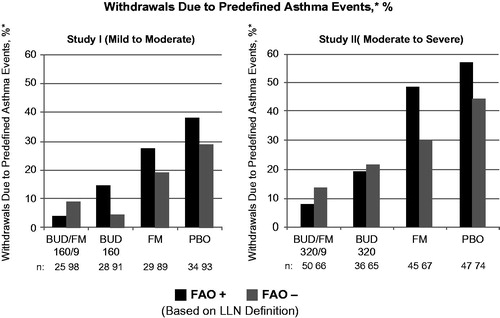
Table 1. Demographic and screening disease characteristics by FAO category (LLN definition).
Table 2. Baseline disease characteristics and control measures by FAO category (LLN definition).
Figure 2. Adjusted* mean changes from baseline in % of asthma control days by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; LLN, lower limit of normal; PBO, placebo.
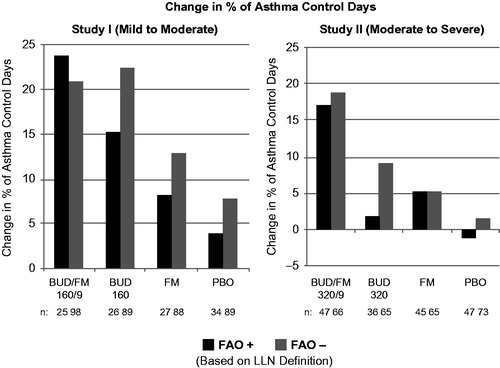
Figure 3. Adjusted* mean changes from baseline in predose FEV1 by FAO category (LLN definition) in study I (mild-to-moderate asthma) and study II (moderate-to-severe asthma). Run-in treatment was placebo for study I and lower dose budesonide for study II (see “Methods” section for run-in and treatment details). *Data presented as least-squares mean unless otherwise noted. BUD/FM, budesonide/formoterol; FAO, fixed airflow obstruction; FEV1, forced expiratory volume in 1 s; LLN, lower limit of normal; PBO, placebo.
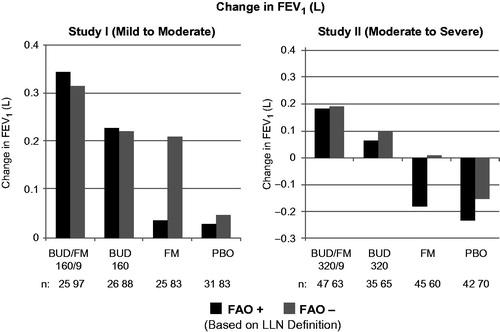
Table 3. Treatment outcomes (adjusteda mean changes from baseline in rescue medication use and the percentage of awakening-free nights) by FAO category (LLN definition).
