Figures & data
Table I. National Cancer Institute’s common terminology criteria versions 1-3 for arterial thromboembolic events.
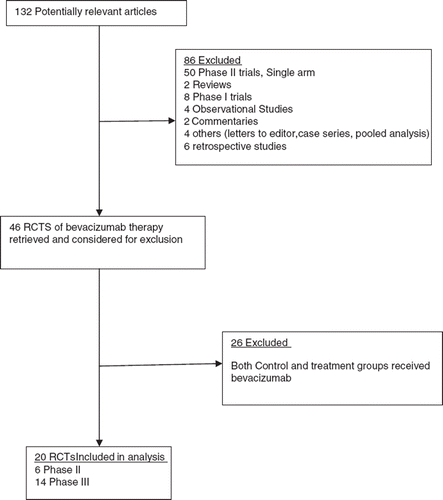
Table II. Characteristics of randomized controlled trials included in the meta-analysis.
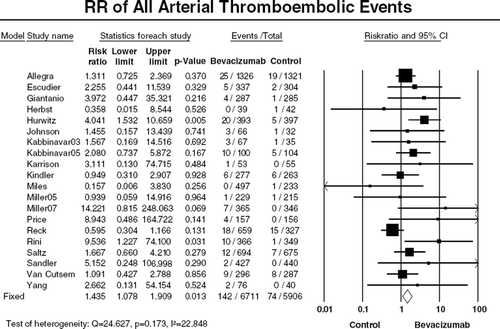
Table III. Incidence and relative risk (RR) of all-grade arterial thromboembolic events (ATE) with bevacizumab according to tumor types.
Table IV. Incidence and relative risk (RR) of high-grade arterial thromboembolic events (ATE) with bevacizumab according to tumor types.
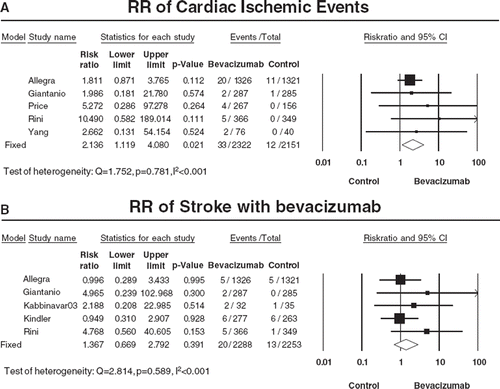
Table V. Incidence and Relative Risk (RR) of arterial thromboembolic events (ATE) with bevacizumab according to organs.