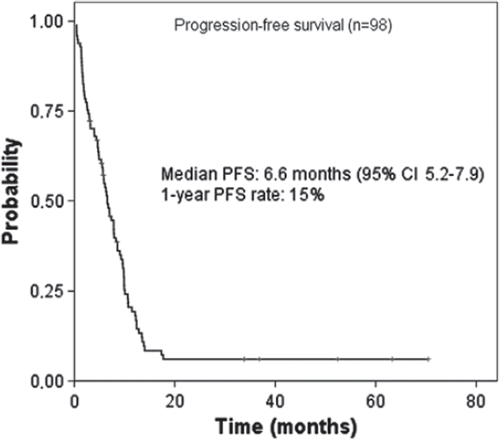
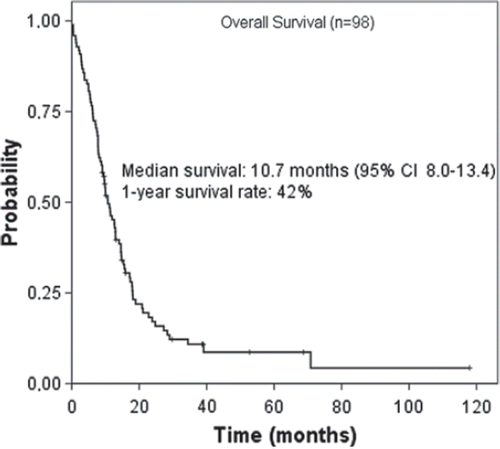
Figures & data
Table I. Patient characteristics (n=98).
Table II. Treatment-related toxicities* (No of courses = 535) - rates in brackets.
Table III. Responses to therapy for patients with measurable disease (n=91).
Table IV. Two-drug regimens containing platinum, taxane or gemcitabine.
Table V. Three-drug regimens containing platinum, taxane and/or gemcitabine.