Figures & data
Figure 1. Apoptosis vs. non-apoptotic mechanisms of programmed cell death. Mammalian cells seem to possess multiple death mechanisms, but it is unclear how much each of these mechanisms contributes to programmed death in vivo.
Table I. Cell death mechanisms observed in cultured cells.
Figure 2. Role of the mitochondria in apoptosis and necrosis. Permeabilization of the mitochondrial outer membrane occurs during apoptosis and results in the release of some apoptogenic molecules, including cytochrome c. Subsequently, cytochrome c is assembled into apoptosomes with Apaf-1 and pro-caspase-9, resulting in the activation of caspases. Oxidative stress or an excess of calcium can trigger the mitochondria membrane permeability transition (MPT), which is initiated by an increase of inner mitochondrial membrane permeability that results in loss of the membrane potential. This is followed by mitochondrial swelling and rupture of the outer membrane with mitochondrial dysfunction and depletion of ATP that causes necrotic death.
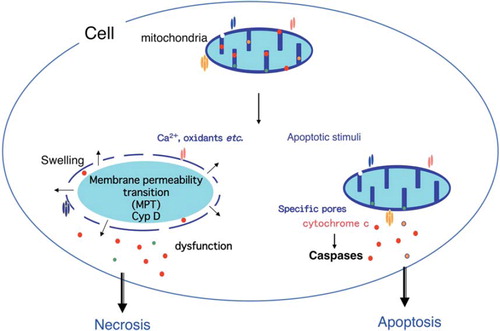
Figure 3. Necroptosis/programmed necrosis. Death receptors can activate two signaling pathways that lead to caspase-8-dependent apoptosis and RIP1/RIP3-dependent necrosis called necroptosis/programmed necrosis. Activated caspase-8 cleaves and inactivates RIP1/3 kinases, shifting the outcome of death receptor activation to apoptosis. When caspase-8 activation is inhibited, for example by a defect of caspase-8 or FADD or by caspase inhibitors, such as zVAD-fmk, cells die of necroptosis instead.
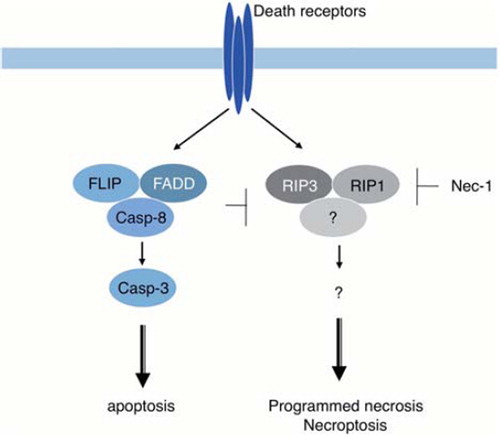
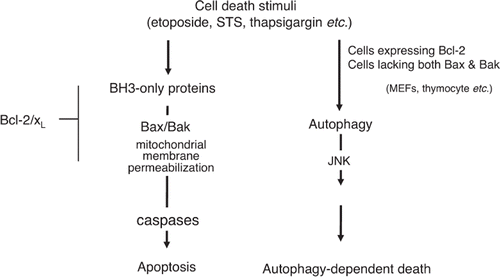
Figure 4. Autophagy-dependent death. Depletion of serum, amino acids, or lymphokines, as well as cytotoxic drugs, such as etoposide and staurosporine, induce apoptosis of wild-type MEFs. When apoptosis is blocked by Bax/Bak-deficiency or overexpression of Bcl-2, however, only etoposide and staurosporine (not withdrawal of amino acids, serum, or lymphokines) trigger autophagy-dependent death. Autophagy-dependent death requires the activation of JNK.