Figures & data
Figure 1. Inclusion/exclusion in study of chemotherapy use in men with castration resistant prostate cancer (PCa) in PCBaSe Sweden. Study inclusion period is the period during which a case had to die of PCa to be included. Observation period in SALT database is the period prior to death during which data on use of chemotherapy was available. Sjukhusapotekens läkemedelstillverkning (SALT)/Hospital pharmaceutical manufacturing database, an in-hospital medication database. Solid lines represents men followed diagnosed with PCa. Grey and black colors used for time with unknown and known chemotherapy status respectively. The solid lines ends in PCa death (dark grey) or death of other causes (light grey). The inclusion criteria are men dead from PCa within the study inclusion period 21 May 2009 and 31 December 2010. PCa diagnosis recorded in NPCR. Cases included: 1) Men who were diagnosed with PCa, registered in NPCR prior to the observation period in SALT database and died from PCa during the study inclusion period; and 2) Men diagnosed with PCa during the observation period in SALT database and died from PCa during the study inclusion period. Cases excluded: 3) Men who died from PCa prior to the study inclusion period; 4) Men who died from other causes during the study inclusion period; and 5) Men still alive at end of the observation period in SALT database.
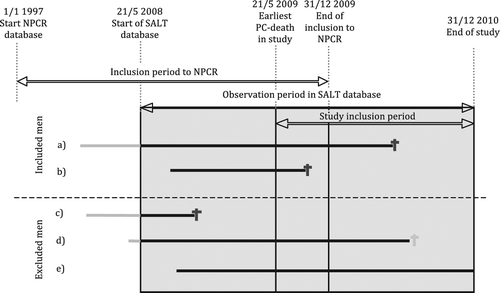
Table I. Clinical and demographic characteristics of men who died from prostate cancer treated or not treated with chemotherapy in PCBaSe Sweden.
Table II. Chemotherapy regimens and timing.
Table III. The 10 most commonly used chemotherapy agents.
Figure 2. The likelihood of initiation of chemotherapy for various subgroups of men who died from PCa 2009–2010 expressed as odds ratios and 95% confidence intervals. For definition of Charlson Comorbidity Index (CCI) and education, see materials and methods section. Primary treatment; other palliative, e.g. transurethral resection of the prostate, steroids, analgesics. Endocrine treatment – Gonadotropin Releasing Hormone analogues (GnRH).
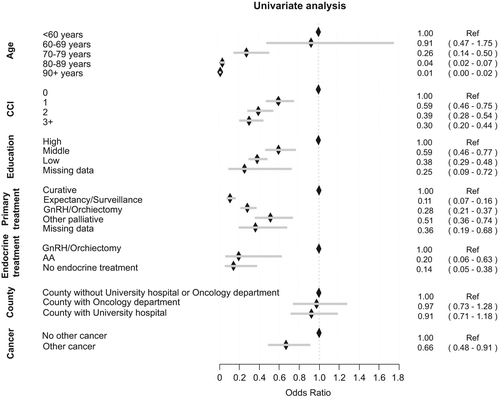
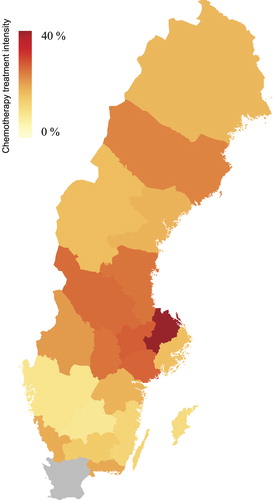
Figure 3. Differences between counties in use of intravenous docetaxel chemotherapy treatment, i.e percentage of men dying from prostate cancer who received intravenous docetaxel chemotherapy prior to death. Data from Skåne county is missing (gray).