Figures & data
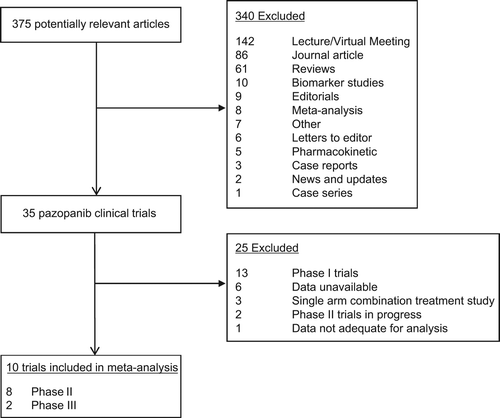
Table I. Characteristics of trials included in the meta-analysis.
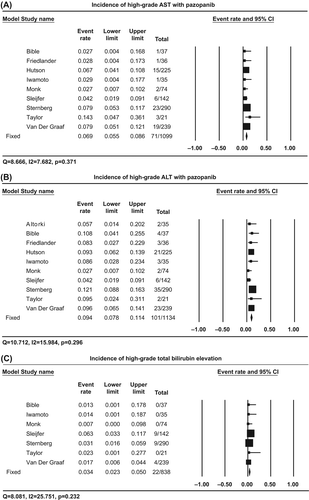
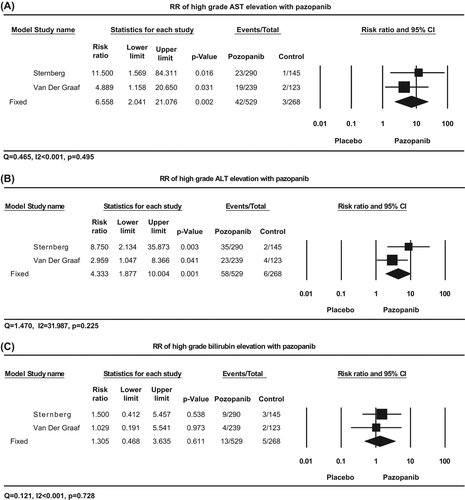
Table II. Incidence and relative risk of liver toxicity with pazopanib.
Table III. Risk of liver toxicity with VEGFR tyrosine kinase inhibitors.