Figures & data
Table I. Patient Characteristics.
Figure 1. Bone resorption marker levels, CTX, in response to Zol treatment. CTX-levels: A) measured in blood samples taken immediately before and 14 days after protocol related infusion of 4 mg Zol according to the treatment groups. Lines represent responses of individual patients within 14 days (statistics: two-sided paired t-test). The insert represents an enlargement to better visualize the individual changes in the MM ≥ 6 group. The means for each before/after measurement were as follows: MM = 0 (n = 10), before = 0.68, after = 0.14; MM ≥ 6 (n = 20), before = 0.083, after = 0.067; BC = 0 (n = 10), before = 0.58, after = 0.13; BC ≥ 6 (n = 17), before = 0.18, after = 0.16. B) Average CTX levels in different patients groups (statistics: two-sided Mann Whitney test). MM = 0, n = 10; MM ≥ 6, n = 20; BC = 0, n = 10; BC ≥ 6, n = 18. C) CTX-levels of each individual patient according to the total number of previous Zol infusions (two-sided Spearman's correlation analysis (rs). MM, n = 30; BC, n = 28. Abbreviations used: a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Some patients were excluded from these analyses as indicated above because one or both blood samples by mistake were not measured according to the protocol guidelines.
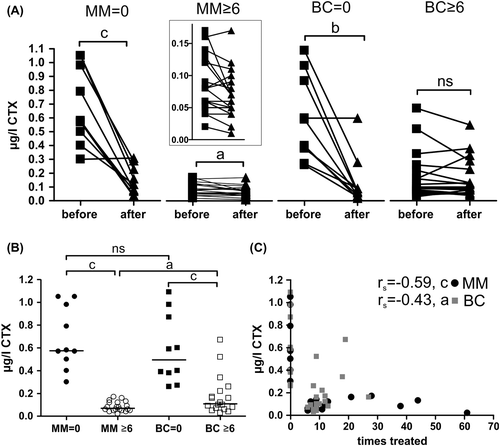
Figure 2. Bone formation marker, bALP, in response to Zol treatment. bALP-levels: A) immediately before and 14 days after protocol related infusion of 4 mg Zol according to the treatment groups. Lines represent responses of individual patients (statistics: two-sided paired t-test). The insert represents an enlargement to better visualize the individual changes in the MM ≥ 6 group. The means for each before/after measurement were as follows: MM = 0 (n = 10), before = 44.9, after = 36; MM ≥ 6 (n = 20), before = 15.5, after = 14.7; BC = 0 (n = 10), before = 65, after = 63.2; BC ≥ 6 (n = 18), before = 33.2, after = 34.6. B) Average bALP-levels in different patients groups (statistics: two-sided Mann Whitney test). Vertical bars represent the median. MM = 0, n = 10; MM ≥ 6, n = 20; BC = 0, n = 10; BC ≥ 6, n = 19. C) bALP-levels of each individual patient according to the total number of previous Zol infusions (two-sided Spearman's correlation analysis). MM, n = 30; BC, n = 29. Abbreviations used: a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Some patients were excluded from these analyses as indicated above because one or both blood samples were not measured according to the protocol.
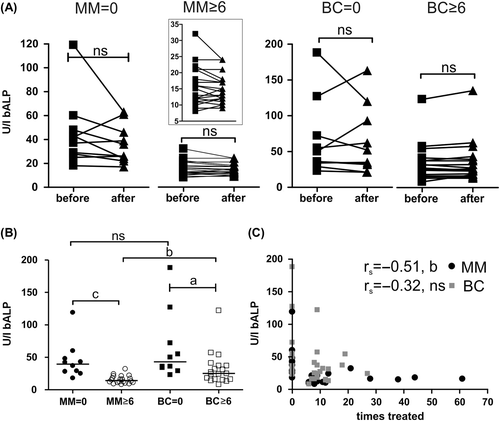
Figure 3. Bone turn-over markers are elevated if patients are not treated regularly every month. A) CTX-levels in patients that have received at least 6 previous treatments and their prior dosing regimen (mg Zol infused/month before). 4 mg/month reflects on average a monthly regimen while 2 mg/month reflects dosing regimen every 2nd month and so forth. MM, n = 30; BC, n = 28. B) bALP-levels, as desribed in A. MM, n = 30; BC, n = 29. C-D) To improve visibility of the data, CTX (C) and bALP (D) data for MM patients are here shown on another scale, but are otherwise the same data as shown in A and B, respectively. E-F) CTX (E) and bALP (F) values for BC patients. Data are the same as shown in A and B, respectively. Statistics: two-sided Spearman correlation; a: p < 0.05, b: p < 0.01, c: p < 0.001, ns: not significant. Lines included represent trend-lines and do not indicate linear correlation. Some patients were excluded from these analyses as indicated above because one or both blood samples were not measured according to the protocol.
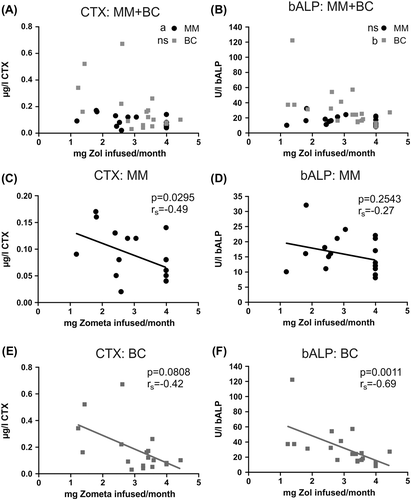
Figure 4. Zol dosing is the best predictor of bone marker levels. A) Dependent variable: CTX. An initial multiple linear regression model of power transformed CTX-levels was generated including seven different variables (in addition to the statistical variable “_cons”: constant or intercept) (table to the left) that could be considered to be of relevance for predicting CTX-levels in BC (n = 27) or MM (n = 30) patients. The table on the right hand side shows the final optimized model containing those variables and corresponding results of the multiple regression that best predict CTX-levels. In between the tables is shown the statistical parameters of the likelihood ratio test. B) Dependent variable: bALP. Same as in A, BC (n = 28) or MM (n = 30) patients. Some patients were excluded from these analyses as indicated above because not all variables included in the model had been determined correctly for all patients. WBrt, whole body retention.
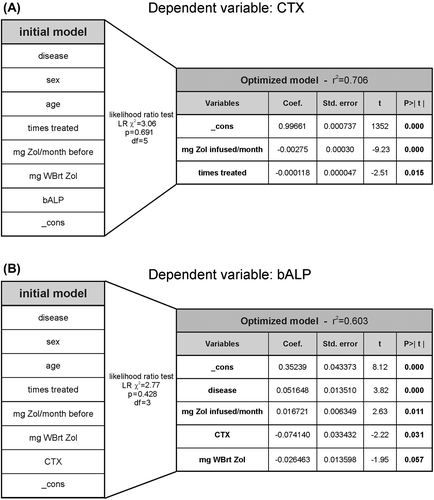
Figure 5. Prolonged Zol treatment negatively affects creatinine clearance in MM patients. A) Average estimated creatinine clearance rates in different patient sub-groups (statistics: two-sided t-test). B) Estimated creatinine clearance rates as a function of the total number of previous Zol treatments (statistics: two-sided Spearman's correlation). C) Estimated creatinine clearance rates as a function of the time (months) from diagnosis of bone disease in MM or BC patients (statistics: two-sided Spearman's correlation). D) Estimated creatinine clearance rates as a function of the age of the patients (statistics: linear regression analysis). P-values: a: p ≤ 0.05, b: p < 0.01, ns: not significant.
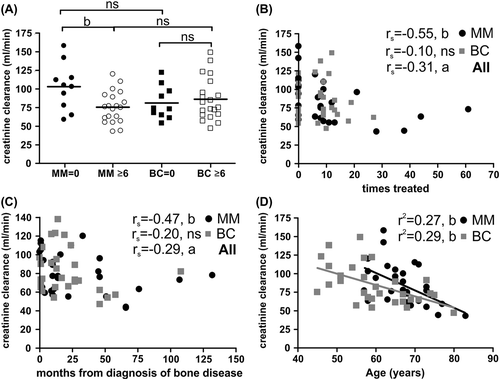
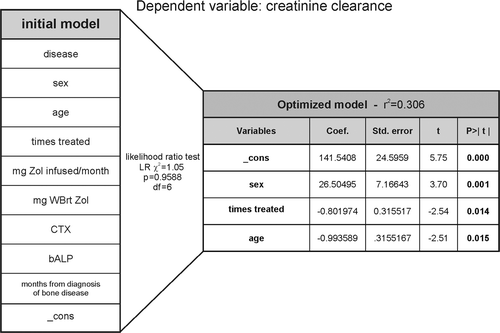
Figure 6. The accumulated number of Zol treatments is a strong predictor of creatinine clearance. Dependent variable: creatinine clearance. An initial multiple linear regression model was generated including 9 different variables (in addition to the statistical variable “_cons”: constant or intercept) (table to the left) that could be considered to be of relevance for predicting creatinine clearance in BC (n = 28) or MM (n = 30) patients. The table on the right hand side shows the final optimized model containing those variables that best predict creatinine clearance together with the statistical results of the multiple regression. In between the tables, is shown the statistical parameters of the likelihood ratio test. WBrt, whole body retention.