Figures & data
Table I. Patient characteristics.
Figure 1. Representative examples of dose distribution for the craniospinal irradiation method. (A) Sagittal view (left: proton beam therapy; right: photon beam therapy). (B) Axial view (top: proton beam therapy, bottom: photon beam therapy). (C) Dose-volume histograms for the brain and spinal planning target volumes, and of specific tissues of interest (solid line: proton beam therapy, dashed line: photon beam therapy). PTV, planning target volume.
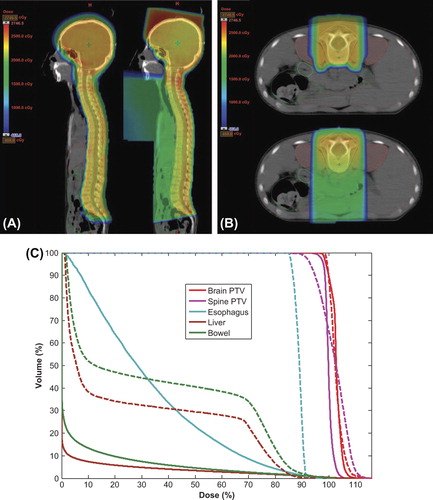
Table II. Acute toxicities.
Figure 2. (A,B) Changes in thrombopoietin levels (A) and platelet counts (B) during craniospinal irradiation. (C,D) Changes in thrombopoietin (C) and platelet counts (D) in five patients who did not undergo chemotherapy after craniospinal irradiation. Values in A and B are means with 95% confidence intervals. Values in C and D are the actual values in each patient.
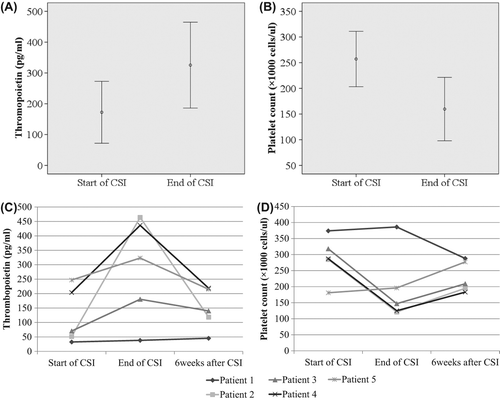
Table III. Changes in haematological parameters from before starting CSI to 1 month after treatment.
