Figures & data
Table I. Baseline patient demographics and clinical characteristics of the entire cohort.
Table II. Possibly/probably related adverse events in phase Ib.
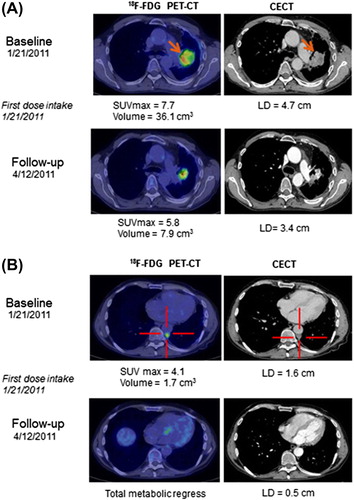
Figure 1. Kaplan-Meier plot for the overall survival of the total population (n = 49) with one patient alive at cut-off.
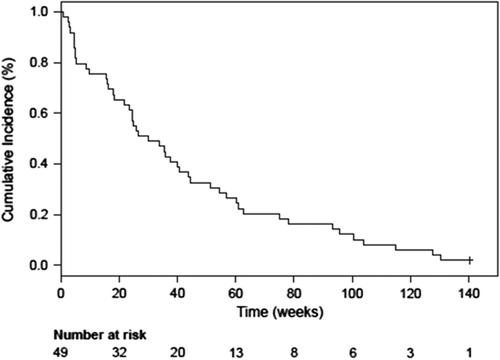
Figure 2. Kaplan-Meier plot for the overall survival (with one patient alive at cut-off) and the progression-free survival of the total population in multidose (Phase Ib) with a treatment duration longer than 2 weeks with single agent AXL1717 (n = 34).
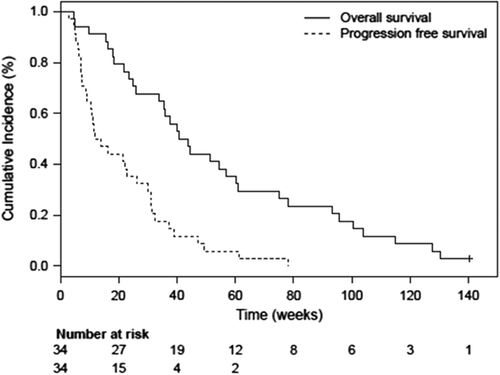
Figure 3. Kaplan-Meier plot for the overall survival (with no patients alive at cut-off) and progression-free survival of the 15 patients with NSCLC with treatment duration longer than 2 weeks with single agent AXL1717.
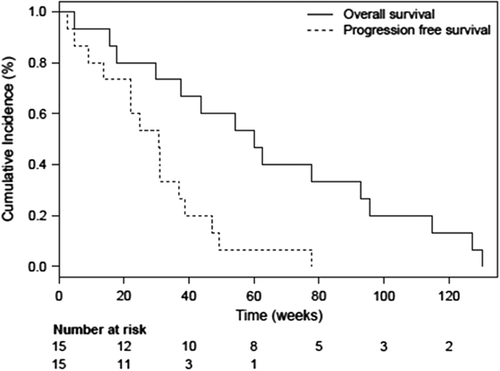
Figure 4. A and B Transaxial images at baseline and 3-month follow-up with FDG PET-CT and contrast-enhanced CT (CECT) in a patient with a primary NSCLC (solid arrows) in the left lung and pleural metastases (cross marked). A) shows a significant reduction in the primary tumor metabolism measured in maximum standardized uptake value (SUVmax, g/mL) and size on CECT evaluated with the RECIST 1.1 criteria (LD, transaxial longest diameter) at 3-month follow-up. B) shows a concordant significant reduction in the pleural metastases with total metabolic regress on FDG PET-CT after treatment.