Figures & data
Figure 1. Schematic representation of TGF-β signal transduction pathways. TGF-β transduces signals through two different types of serine/threonine (and tyrosine) kinase receptors, termed TβRI and TβRII. Upon TGF-β binding, TβRI and TβRII form heterotetrameric complexes, and TβRII kinase transphosphorylates the juxtamembrane portion (GS domain) of the cytoplasmic region of TβRI. Phosphorylated TβRI transmits intracellular signaling through R-Smad phosphorylation. Smad2 and Smad3 are R-Smads phosphorylated by TβRI kinase and form heteromeric complexes with Smad4 (co-Smad). Smad complexes translocate into the nucleus and act as transcriptional regulators of target genes by interacting with other transcription factors and transcriptional regulators. Smad7 (I-Smad), which lacks the typical MH1 domain, interferes with the activation of R-Smads by interacting with TβRI and competitively prevents R-Smads from being phosphorylated by TβRI. TGF-β activates other intracellular signaling pathways in addition to Smads in order to regulate a wide array of cellular functions. These non-Smad pathways are activated by TGF-β receptors through phosphorylation or direct interaction.
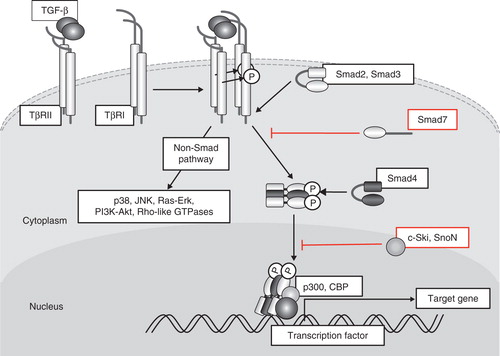
Figure 2. Schematic representation of EMT induction by TGF-β and FGF-2. ‘Epithelial cells’ differentiate into ‘fibroblastic cells’ through EMT induced by TGF-β and further differentiate into α-SMA-positive ‘myofibroblastic cells’ through epithelial-myofibroblastic transition (EMyoT). When FGF-2 is present in this process, FGF-2 induces differentiation of epithelial cells to ‘activated fibroblastic cells’.
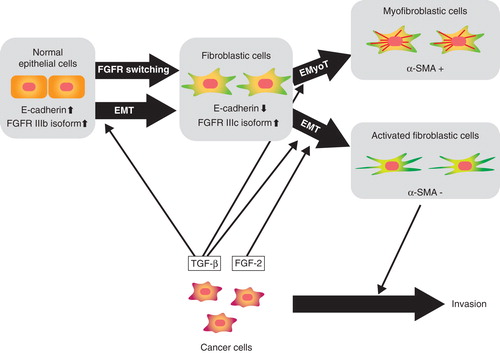
Figure 3. Mechanisms of TGF-β action on prevention of breast cancer metastasis using JygMC(A) cells. Endogenously activated autocrine loop of TGF-β regulates the expression of E-cadherin and N-cadherin by inducing EMT in JygMC(A) cells. Autocrine TGF-β also regulates the expression of various transcription factors, including Dec1 and Foxc1, and promotes the survival. Negative regulators of TGF-β signaling (Smad7, c-Ski, or TGF-β inhibitors) block these pathways and inhibit metastasis of JygMC(A) cells.
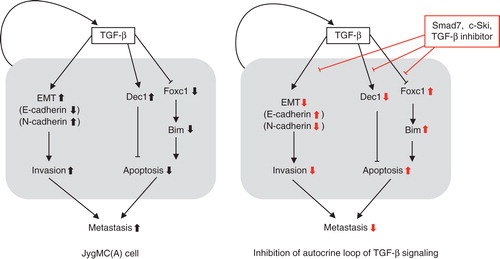
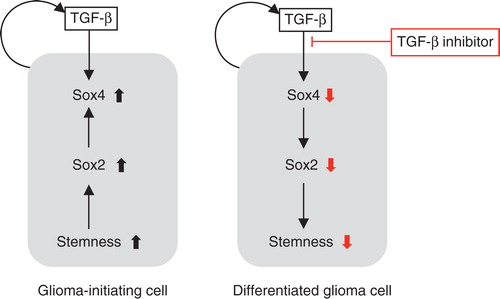
Figure 4. Effects of the TGF-β–Smad–Sox4–Sox2 axis on the maintenance of GIC stemness. TGF-β directly induces Sox4 expression. Subsequently, Sox4 promotes Sox2 expression, which plays significant roles in sustaining GIC stemness. TGF-β inhibitor blocks this TGF-β–Sox4–Sox2 axis, promotes GIC differentiation, and deprives these cells of their aggressiveness. Differentiated glioma cells (right panel) may be more sensitive to conventional chemotherapy and radiotherapy than undifferentiated GICs (left panel).