Figures & data
Figure 1. CAF-derived factors can modulate tumor development and progression. The figure schematically illustrates various aspects of tumor initiation progression and metastasis where fibroblasts have been indicated as key regulators. Fibroblasts play a role in tumor initiation and the transition of ductal carcinoma in situ towards invasive carcinoma. During tumor progression, CAF-released growth factors and cytokines promote the proliferation of stem cell-like/mesenchymal cells. By the release of proteases and force-mediated remodeling of the extracellular matrix CAFs also support the migration of tumor cells. Activation of fibroblasts is an important component in the formation of pre-metastatic niches.
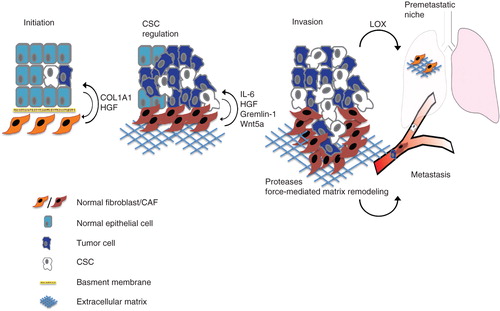
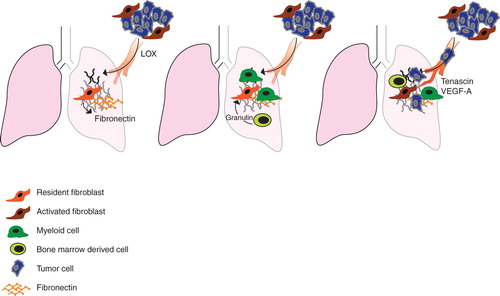
Figure 2. Fibroblast involvement in the formation of a metastasis-permissive microenvironment. A: Local fibroblasts respond to systemic signals by depositing fibronectin in the target organ and thereby priming it for homing of myeloid cells and subsequent metastatic settlement. In an alternative pathway of metastatic initiation, LOX is secreted from the primary tumor to cross-link collagen IV in fibronectin-rich areas of the target organ. B: The ‘primed' microenvironment of the pre-metastatic site promotes recruitment of myeloid cells and subsequently also bone-marrow-derived cells and tumor cells. C: In response to systemic signaling, mobilized bone-marrow cells are recruited to the secondary site. Through a granulin-dependent mechanism they can then activate local fibroblasts. Presence of activated fibroblasts, producing VEGF-A and tenascin, supports angiogenesis in metastasis growth.