Figures & data
Figure 1. Schematic illustration of AMP interaction with lipid membranes. In barrel-stave pores, peptide oligomers organize in a trans-membrane structure, while toroidal pores are disorganized membrane defects caused by curvature strain. Higher peptide densities may subsequently cause complete membrane disintegration (micellization). Furthermore, peptide binding to the polar headgroup region allows relaxation of the alkyl chains and causes membrane thinning. In addition, chemical potential gradients may result in peptide translocation across the membrane. Finally, peptide-induced lipid segregation or phase separation may contribute to AMP-induced membrane rupture.
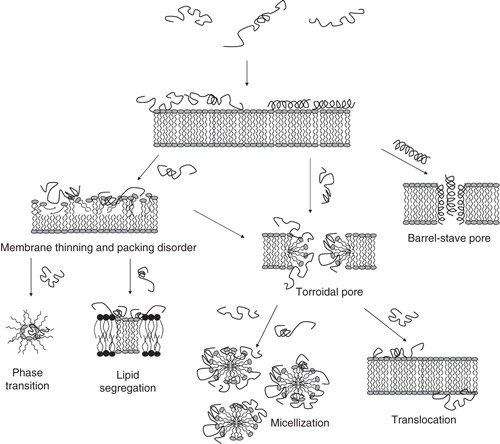
Figure 2. Combined hemolysis (A) and viable count (VCA), (B) assay for S. aureus and P. aeruginosa (both 2 × 108 cfu/mL) added to 50% citrate blood at peptide concentrations of 60 and 120 μM (Citation23).
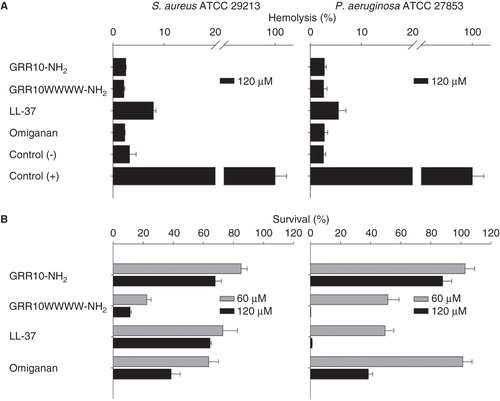
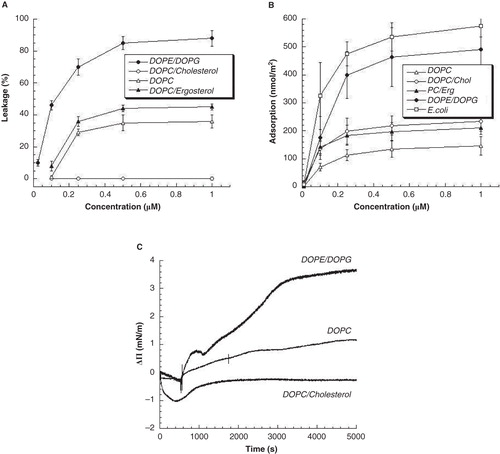
Figure 3. (A) Peptide-induced liposome leakage for GRR10W4N at 10 mM Tris, pH 7.4. Shown in (B) and (C) is the adsorption of the same peptide to supported lipid bilayers, as well as the change in surface pressure (ΔΠ) due to insertion of GRR10W4N to zwitterionic DOPC and DOPC/cholesterol, and anionic DOPE/DOPG monolayers from Tris buffer, pH 7.4 (Citation23).