Figures & data
Figure 2. Countries that had notified at least one case of XDR-TB by the end of 2012. Figure reproduced with kind permission from the World health Organization (Citation12).
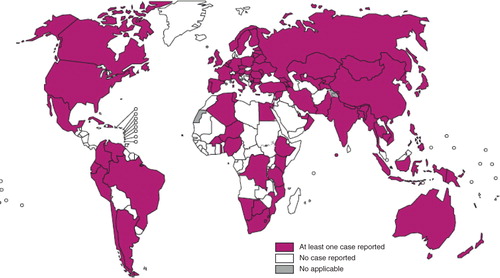
Figure 4. Preparation of new fluoroquinolones via microwave assisted Suzuki cross-coupling reaction.
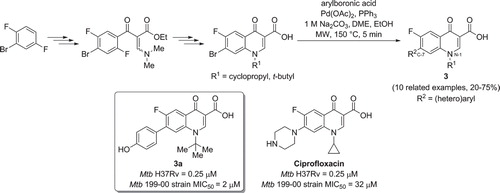
Figure 5. a) Microwave assisted preparation of a Mtb inhibitor via Fischer indolization. b) Synthesis of Mtb inhibitors via a microwave assisted ammonolysis.
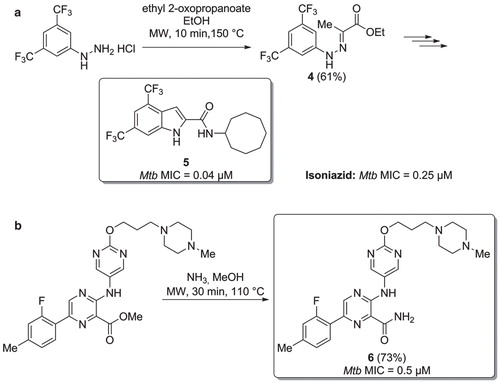
Figure 6. a) L-methionine-(SR)-sulfoximine (MSO). b) Synthesis of Mtb-GS inhibitors via a multi-step microwave assisted synthetic route. c) Microwave assisted synthesis of Mtb-GS inhibitors via multi-component heterocyclization.
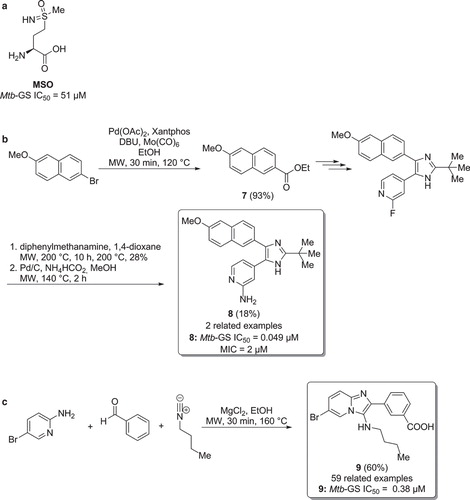
Figure 7. a) Structure of fosmidomycin. b) Synthesis of fosmidomycin analogues. c) Synthesis of Mtb-IspC inhibitors via a microwave promoted oxidative Heck reaction. d) Synthesis of Mtb inhibitors via a heterocyclization.
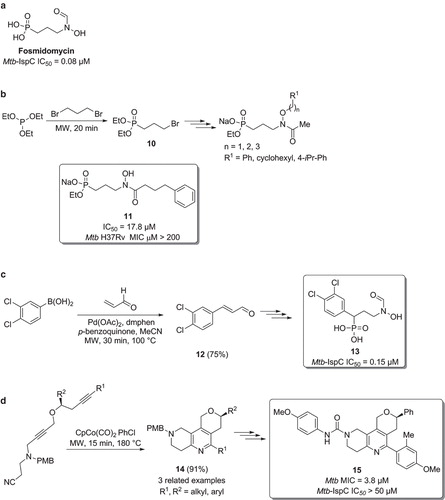
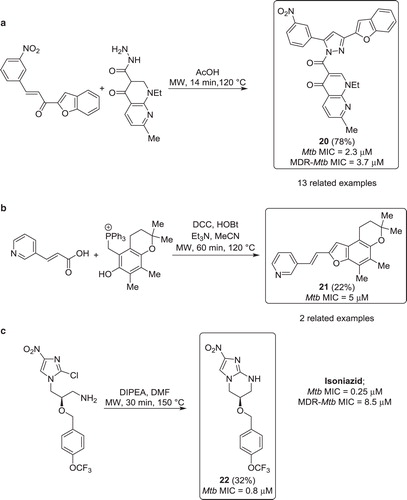
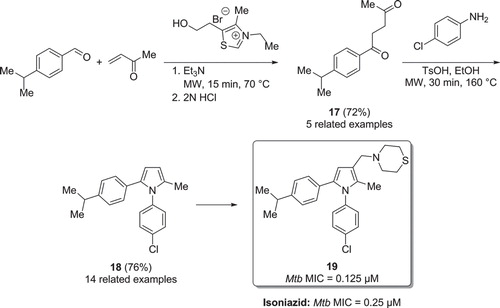
Figure 10. a) Synthesis of Mtb inhibitors via a microwave heated 5-membered heterocyclisation. b) Synthesis of Mtb inhibitors via a microwave assisted Wittig reaction. c) Synthesis of Mtb inhibitors via a microwave promoted intramolecular SNAr reaction.