Figures & data
Figure 1. Actual and predicted plasma concentration – time profiles for 20 mg intravenous (IV) and 50 mg oral administrations of melperone.
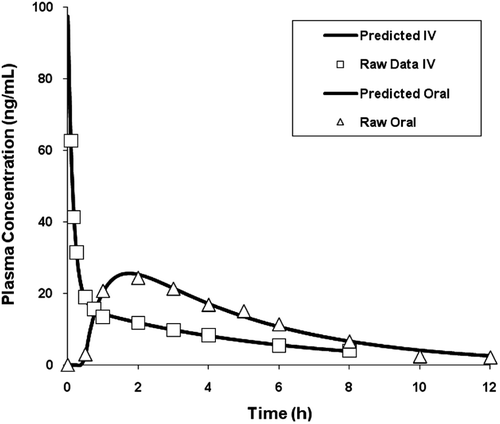
Table 1. Paramater estimates of 20 mg I.V. and 50 mg P.O. melperone.
Figure 2. Schematic of the 2-compartment PK modeling for melperone based on first order kinetics of drug release from SR beads into the environment of use, systemic absorption of the released drug, and distribution and elimination of the absorbed drug.
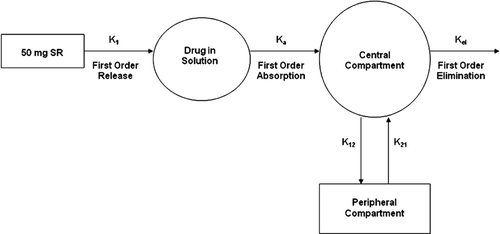
Figure 3. Predicted in vitro melperone release profiles of prototype once-daily dosage forms: (A) fast, medium, or slow release dosage forms, 25 mg and (B) fast, (C) medium, and (D) slow release dosage forms, 25 and 50 mg.
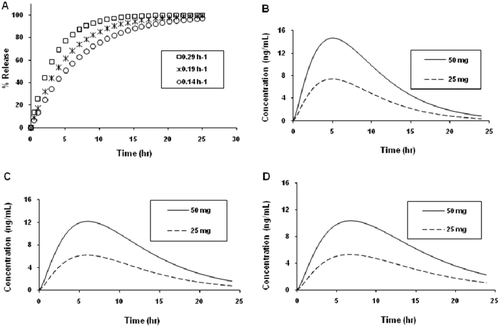
Table 2. Time for release of 95% of the drug load (T95).
Table 3. AUC of melperone at different release rates.
Table 4. Solubility (mg/mL) of melperone HCl.
Figure 4. Schematic of SR bead (Diffucaps® bead comprising an inert core sequentially coated with a drug layer, a protective seal coat, an alkaline buffer layer, and an outer SR polymer coat).and CR Capsules and ODT tablet comprising SR beads.
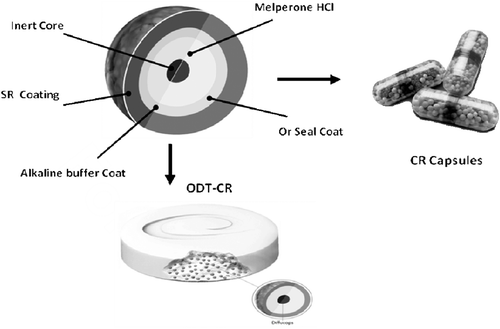
Figure 5. Melperone HCl release profiles from prototype SR bead formulations. Figure shows theoretical fast and slow release profiles and melperone release profiles from prototype SR beads coated at 15, 20, 25, and 30% by weight of the SR beads.
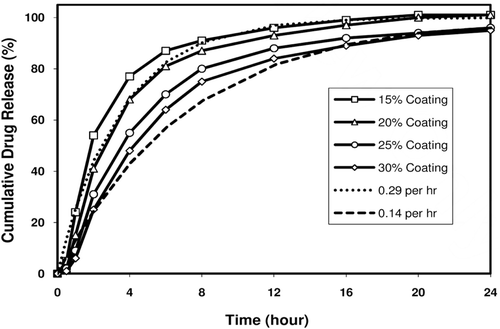
Figure 6. Melperone HCl release profiles from prototype ODT-CR formulations. Figure shows similar melperone release profiles from prototype ODT CR 161 and ODT CR 55 together with the corresponding SR beads.
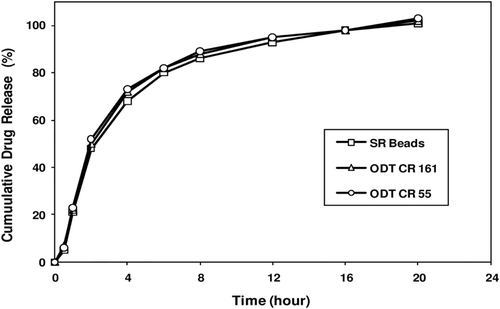
Table 5. Compositions and tableting properties of ODTs.
Figure 7. Melperone HCl release profiles from prototype CR Capsule formulations. Figure shows varying release profiles from fast, medium, and slow release capsule formulations containing SR beads coated at different levels.
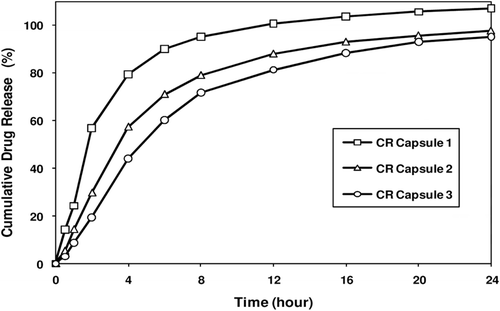
Figure 8. In vitro drug release profiles of pilot CTM supplies – ODT-CR (Test 1 (fast release) & Test 2 (medium release) contain an alkaline buffer layer and smaller inert cores) and CR Capsule (Test 3 contain larger inert cores without an alkaline buffer layer).
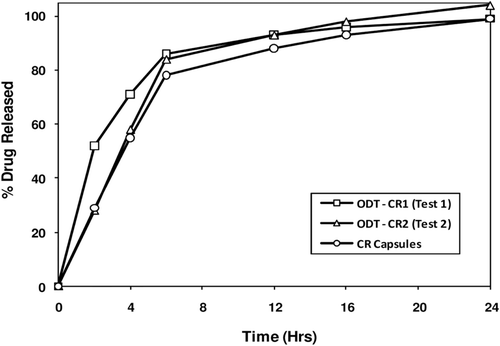
Figure 9. Plasma concentration-time profiles of pilot CTM supplies – Syrup 25-mg, ODT-CR (Test 1 (fast release) & Test 2 (medium release)), and CR Capsule (Test 3).
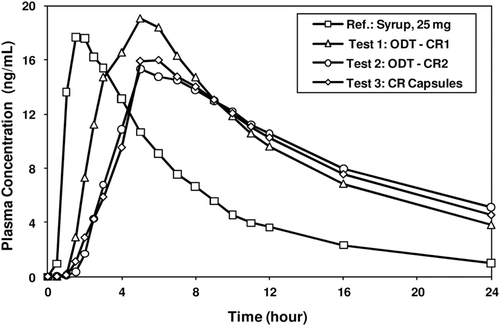
Table 6. PK parameters for melperone treatments A (25-mg Syrup; 50-mg Syrup), B (50-mg ODT-CR 1), C (50-mg ODT-CR 2), and D (50-mg CR Capsule).
Table 7. Melperone plasma PK data - least-squares means and 90% CIs.
Table 8. Stability data for Melperone CR Capsules and ODT-CR 2.
Figure 10. Drug release profiles of ODT formulations and corresponding SR beads manufactured at one-tenth commercial scale. Figure shows minimum membrane fracture when compressible coated SR beads tableted with rapidly dispersing microgranules at different ratios of SR coated beads to RD microgranules, ODT tablets with a higher fraction of RD microgranules providing slightly faster release.
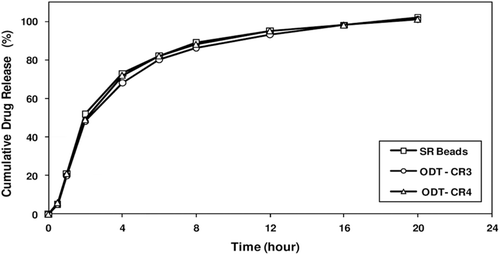
Table 9. Compositions and tableting properties of ODTs.