Figures & data
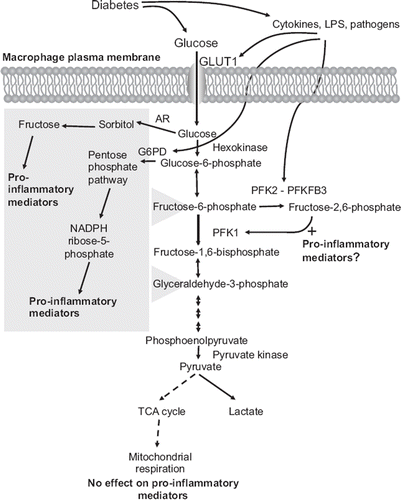
Figure 1. Schematic representation of the potential links between diabetes, glucose metabolites, and pro-inflammatory effects in macrophages. Macrophages rely heavily on glycolysis as an energy source, both under aerobic and anaerobic conditions. Diabetes is likely to increase glucose uptake in macrophages. Cytokines and pathogens also stimulate glycolysis by increasing expression of GLUT1, G6PD, and PFKFB3. Diabetes could therefore potentially stimulate glucose flux in macrophages through two mechanisms, by a direct effect through hyperglycemia, and by promoting a pro-inflammatory environment, which in turn further enhances glucose metabolism in a self-perpetuating cycle. Recent research shows that the pro-inflammatory phenotype of macrophages associated with diabetes might be due to increased glucose flux through the polyol/sorbitol pathway, the pentose phosphate pathway, and/or glycolysis, whereas mitochondrial respiration does not appear to affect the pro-inflammatory phenotype. The polyol/sorbitol pathway and pentose phosphate pathway both feed into glycolysis (as indicated by the gray box) and therefore could contribute to the increased flux through glycolysis.