Figures & data
Table I. Key data on cervical cancer, HPV-16/18 prevalence, and cervical cancer prevention strategies in 22 countries. Data sourced from the World Health Organization (WHO)/Institut Catala d'Oncologia (ICO) Information Centre on HPV and cervical cancer (Citation105).
Table II. Summary of adverse reactions (ADRs) from HPV vaccines Gardasil and Cervarix. Note that the US FDA Code of Federal Regulation defines a serious adverse drug event as ‘any adverse drug experience occurring at any dose that results in any of the following outcomes: death, a life-threatening adverse drug experience, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant disability/incapacity, or a congenital anomaly/birth defect’ (Citation106).
Figure 1. The rate of adverse reactions (ADRs) from Gardasil and Cervarix reported through various government-official vaccine surveillance programmes. For the data source, see
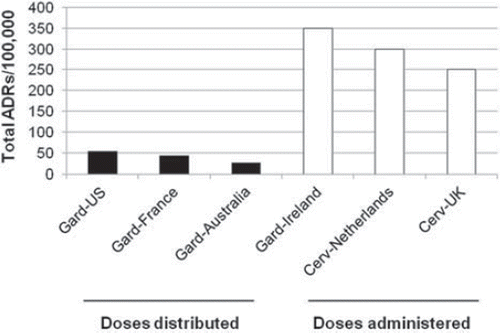
Figure 2. The rate of adverse reactions (ADRs) from Cervarix compared to that of other vaccines in the UK immunization schedule. Data sourced from the report provided by the UK Medicines and Healthcare products Regulatory Agency (MHRA) for the Joint Committee on Vaccination and Immunisation in June 2010 (Citation23).
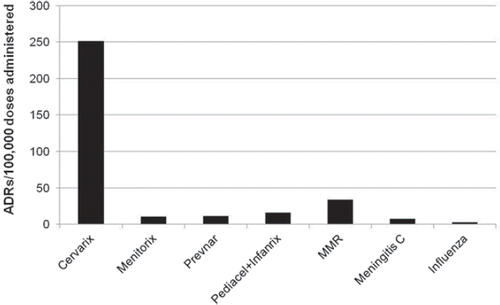
Figure 3. Percentages of reported ADRs associated with HPV vaccines for each system organ class. Data sourced from the Database of the Netherlands Pharmacovigilance Centre Lareb (Citation32), the UK Medicines and Healthcare products Regulatory Agency (MHRA) (Citation62), and the Irish Medicines Board (IMB) (Citation24). The most commonly reported ADRs in the nervous system and psychiatric disorders class were headache, syncope, convulsions, dizziness, hypoaesthesia, paraesthesia, lethargy, migraine, tremors, somnolence, loss of consciousness, dysarthria, epilepsy, sensory disturbances, facial palsy, grand mal convulsion, dysstasia, dyskinesia, hallucination, and insomnia.
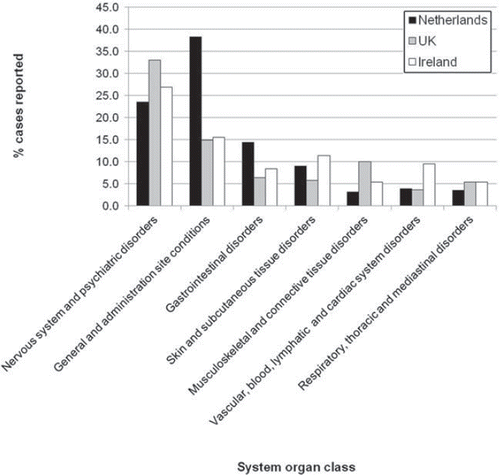
Table III. Injectionsite adverse reactions (ADRs) reported in Gardasil clinical trials among 8878 female participants aged 9–26 years, 1–5 days post-vaccination (Citation82).
Table IV. Number of girls and women aged 9–26 years who reported a condition potentially indicative of a systemic autoimmune disorder after enrolment in Gardasil clinical trials (Citation82).
Table V. Key cervical cancer statistics according to the 2010 World Health Organization (WHO)/Institut Catala d'Oncologia (ICO) report on HPV and related cancers (Citation107).
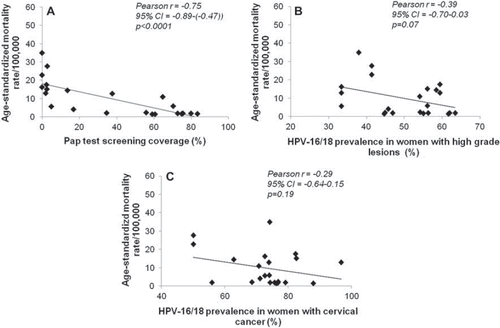
Figure 4. Correlation between cervical cancer mortality rates and A: Pap test screening coverage; B:HPV-16/18 prevalence in women with high-grade lesions (CIN 2/3, carcinoma in situ (CIS), and high-grade cervical squamous intraepithelial lesions (HSIL)); C: HPV-16/18 prevalence in women with cervical cancer. Data were sourced for 22 countries from World Health Organization (WHO)/Institut Catala d'Oncologia (ICO) Information Centre on HPV and cervical cancer (). The correlation analysis was carried out using GraphPad Prism statistical software to derive Pearson correlation coefficients (r). The level of significance was determined using a two-tailed test. The correlation was considered statistically significant at P <0.05.