Figures & data
Table I. Sequence of diff erently tagged versions of human GDNF and primary structure of FLAG-tagged human prepro(α)GDNF.
Figure 1. Characterization of the human prepro(α)GDNF variants. A: Characterization of differently tagged versions of GDNF. CHO cells were transiently transfected with eight constructs encoding different variants of human GDNF or empty vector. The constructs are numbered as in and shown on top of the panel. Two days after transfection, samples were taken from the media and analysed by Western blot using antibodies to GDNF. B: Comparison of cell lines and expression time. PreproFLAG-GG-GDNF (construct number 1, prepro) and preFLAG-GG-GDNF (construct number 5, pre) were chosen for further studies. The two constructs were expressed for 1 and 2 days after transfection (DAT) in HEK 293 and CHO cells. Media were collected and analysed as in (A). C: Comparison of expression media. CHO cells were transfected with preproGDNF (*, no tag), preproFLAG-GG-GDNF (construct number 1, prepro), or preFLAG-GG-GDNF (construct number 5, pre). These constructs were expressed for 2 days in CHO cells grown in DMEM with 10% FBS (DMEMFBS) or Opti-MEM (OM). The media as well as cell lysates (cells lysed in a volume equal to the collected media) were collected and analysed as in (A). D: RET-P activity assay with human prepro(α)GDNF variants. CHO cells were transfected with GFP or the GDNF variants. Constructs number 1–2 (prepro F), number 5–6 (pre F) and number 7–8 (pre H) are numbered according to . Two days after transfection the media were collected and used to stimulate the phosphorylation of RET in MG87-RET cells. Medium from GFP expressing CHO cells was used as a negative control (–). As a positive control, GDNF from E. coli (100 ng/mL) was added to the media from GFP-expressing CHO cells (GDNF/E. coli). All inductions were done in the presence of soluble GFRα1 (1 μg/mL) for 1 hour.
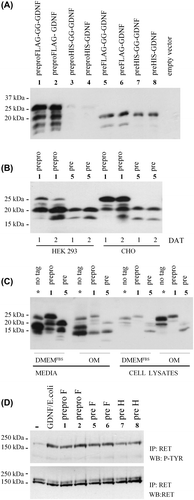
Figure 2. Characterization of N-terminal sequences. Coomassie-stained PVDF membrane of human GDNF electroblotted from reducing SDS-PAGE for N-terminal sequencing. Untagged preproGDNF (-FLAG) as well as preproFLAG-GG-GDNF (+ FLAG) were expressed in CHO cells grown in suspension. The proteins were partially purified, and fractions (Citation1–6) from the heparin column were run on a 15% reducing SDS-PAGE, blotted onto PVDF, and stained with Coomassie brilliant blue. The strong band (I) in lane 3 of the untagged sample, and the two strongest bands (II and III) in lane 3 of the FLAG-tagged sample were excised and the N-terminal sequences of the proteins were determined by Edman degradation.
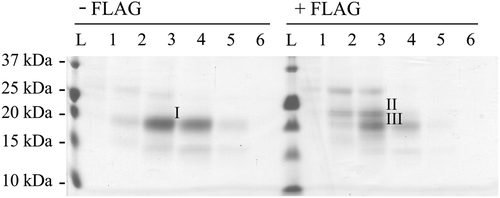
Figure 3. Characterization of N-linked glycosylation. A: Use of the potential N-linked glycosylation sites. CHO cells were transfected with GFP, preproFLAG-GG-GDNF (WT), preproFLAG-GG-GDNFN85A (N85A), or preproFLAG-GG-GDNFN49A (N49A). The cells were incubated in the absence or presence of tunicamycin (TM). After 2 days the media (upper panel) and cell lysates (lower panel) were collected and analysed as in Figure 1A. B: Characterization of unglycosylated GDNF from mammalian cells. PreproFLAG-GG-GDNF (WT), unglycosylated preproFLAG-GG-GDNFN49A (N49A), preFLAG-GG-GDNF (WT w/o pro), and preFLAG-GG-GDNFN49A (N49A w/o pro) were transiently expressed in CHO cells for 2 days and analysed by Western blot analysis with antibodies to proGDNF (Citation18) (upper panel). The filter was stripped and reprobed with antibodies to GDNF (lower panel). Untagged and unglycosylated GDNF from E. coli was included as a control.
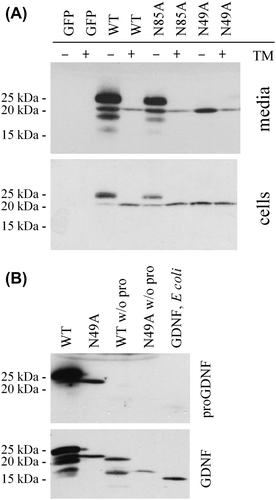
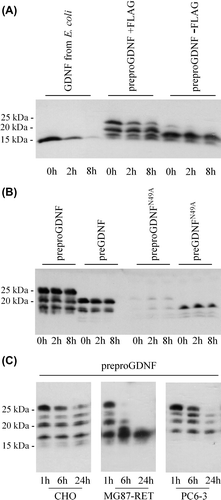
Figure 4. Stability of GDNF from E. coli and mammalian cells. A: Stability of GDNF from E. coli compared to tagged and untagged GDNF from mammalian cells. CHO cells were transfected with untagged GDNF (preproGDNF-FLAG), tagged GDNF (preproGDNF + FLAG), or GFP. After 2 days expression the media were collected and GDNF from E. coli was added to the media from cells expressing GFP. Samples were taken from the media after 0, 2, and 8 hours’ incubation at 37°C, and analysed by Western blot using antibodies to GDNF. B: Stability of unglycosylated GDNF from mammalian cells. CHO cells were transfected with four different versions of FLAG-tagged GDNF: preproFLAG-GG-GDNF (preproGDNF), preFLAG-GG-GDNF (preGDNF) or preproFLAG-GG-GDNFN49A (preproGDNFN49A) and preFLAG-GG-GDNFN49A (preGDNFN49A). After 2 days of expression the media were collected and incubated for 0, 2, and 8 hours at 37°C. The samples were analysed as in (A). C: Stability of mammalian GDNF in different extracellular environment. CHO cells were transfected with preproFLAG-GG-GDNF (preproGDNF). After 2 days of expression (in DMEM with 10% FBS) the media were collected, diluted 1:5 into Opti-MEM , and transferred onto untransfected CHO cells, MG87-RET cells, or PC6 - 3 cells. Samples were taken from the media after 1, 6, and 24 hours’ incubation, and analysed as in (A).