Figures & data
Figure 1. Mechanisms of arrhythmogenesis in long QT syndrome. A: ECG of patient with LQTS degenerating into torsades de pointes (TdP) and showing classical undulating baseline. B: (i) APDs in control and LQTS human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) showing significant prolongation. (ii) ECG in patient with LQTS demonstrating significant increase in QT duration. C: (i) Early after-depolarizations (EADs) in stem cell-derived ventricular and atrial cells. (ii) EADs resulting in triggered activity in iPSC-CMs. D: 12-lead ECG from a patient with LQT2 showing profoundly bifid T waves. Reproduced with permission from (A, B(ii)) Salama and London, J Physiol 2007;578:43–53 (Citation26); (B(i), C) Itzhaki et al., Nature 2011;471(7337): 225–9 (Citation19); (D) Zhang et al., Circulation 2000;102(23):2849–55 (Citation140).
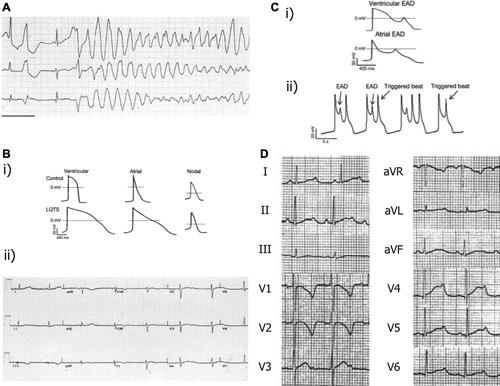
Table I. 2011 LQTS diagnostic criteria.
Table II. Non-structural disorders associated with ventricular arrhythmia and sudden death.
Figure 2. Mechanisms of arrhythmogenesis in Brugada syndrome. A: (i) Two different episodes of polymorphic VT, and (ii) ventricular fibrillation documented in BrS patients with ICDs. B: Precordial leads of a resuscitated patient with BrS. There are dynamic ECG changes in the course of a few days, with all three repolarization patterns shown. Arrows denote the ST elevation. The left panel shows a clear type 1 ECG. Between 7–2–99 and 13–2–99, types 2 and 3 are shown. C: Unipolar electrograms from selected electrodes of a pathfinder catheter (Path1–3) positioned in the great cardiac vein to record from the RVOT epicardium, and of a basket catheter (B2-B8) positioned to record from the RVOT endocardium, after a 20 mg pilsicainide injection. The last beat of constant atrial pacing at a cycle length of 600 ms and a subsequent sinus beat are shown. The second beat shows dramatic abbreviation of activation recovery interval (ARI) in pathfinder electrodes (arrows), whereas ARI in the basket electrode did not change, demonstrating increased dispersion of ARI between the epicardium and endocardium of the RVOT. D: Conduction delay in the RV; activation maps are shown for the RV and left ventricular endocardia. In both panels the black lines represent the septal borders, the dots represent the electrode placement, and the colored isochrones indicate 10 ms increments in activation time. E: Monophasic APD alternans recorded in the RV endocardium of a patient with BrS, followed by induction of ventricular fibrillation by extra-stimuli. Reproduced with permission from (A(i)) Brugada et al., Europace 1999;1:156–66 (Citation141); (A(ii)) Priori et al., J Am Coll Cardiol 2012;59(1):37–45 (Citation142); (B) Wilde et al., Circulation 2002;106:2514–9 (Citation65); (C) Shimizu et al., J Cardiovasc Electrophysiol 2001;12(12): 1418–21 (Citation70); (D) Coronel et al., Circulation 2005;112(18):2769–77 (Citation73); (E) Kofune et al., Circ J 2009 Mar;73(3):580–3 (Citation76).
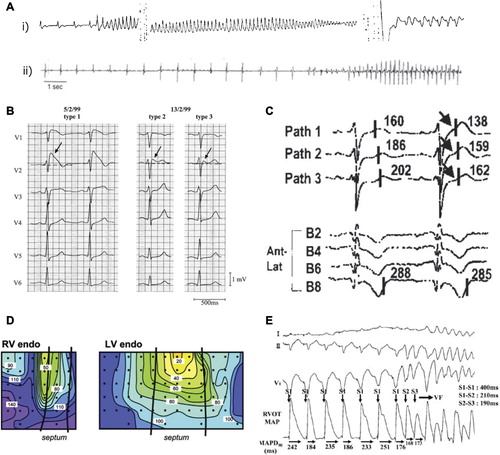
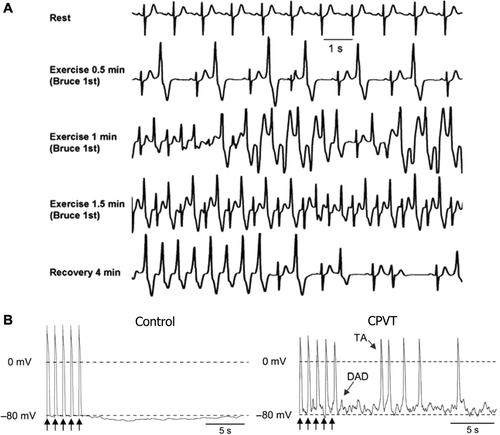
Figure 3. Mechanisms of arrhythmogenesis in catecholaminergic polymorphic ventricular tachycardia. A: Exercise stress test in a patient with polymorphic VT and RyR2 mutation. Ventricular arrhythmias are observed with a progressive worsening during exercise. Typical bidirectional VT develops after 1 minute of exercise with a sinus heart rate of approximately 120 beats per minute. Arrhythmias rapidly recede during recovery. B: AP recordings from control and CPVT stem cell-derived ventricular myocytes. Arrows represent 1 Hz pacing procedure. CPVT shows delayed after-depolarizations (DAD) followed by triggered activity (TA), which is absent in the control. Reproduced with permission from (A) Liu et al., Prog Cardiovasc Dis 2008;51:23–30 (Citation97); (B) Jung et al., EMBO Mol Med 2012;4(3):180–91 (Citation20).