Figures & data
Figure 1. Timeline of access to anti-amyloid therapies for patients with hereditary transthyretin amyloidosis with polyneuropathy. OLE = open-label extension; Ph = phase; Vyndaqel = trade name for tafamidis.
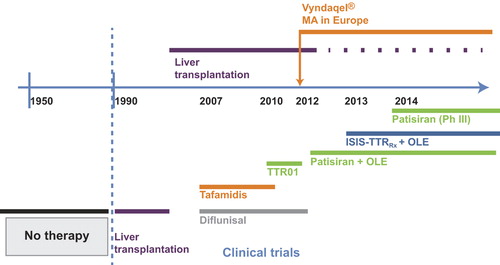
Table I. Key clinical data for novel agents in clinical development of TTR amyloidosis.
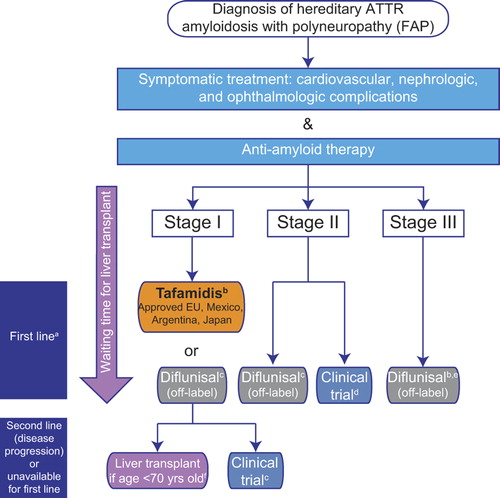
Figure 2. Current treatment pathway for patients with ATTR amyloidosis with polyneuropathy. Disease is classified in stages according to Coutinho et al. (Citation51) at initial diagnosis. a First-line anti-amyloid therapy is initiated according to stage of the disease and approval of the medicine in the country, in parallel with symptomatic treatment, to prevent disease progression and improve patient quality of life. Currently approved treatments may stabilize disease (liver transplantation (Citation53)), or slow progression of the disease (tafamidis, diflunisal (Citation52,Citation96)); treatment options are very limited for patients with stage II and III. The pathway described is followed irrespective of TTR gene mutation at stage I, and initiation of treatment for non-neurologic symptoms (renal, cardiac) may also need to be considered in affected patients. bMostly performed in patients with early-onset FAP with Val30Met mutation. Approval based on pivotal phase II/III study in patients with FAP stage I with Val30Met mutation (Citation96) and an open-label phase II study in patients with FAP stage I with non-Val30Met mutations (Citation97). cDiflunisal should be used with caution in patients with a history of gastrointestinal bleeding or ulceration, renal impairment, or heart failure. dPreliminary data from a phase II open-label extension study with patisiran, a RNAi investigational agent, demonstrate a mean 2.5-point decrease in mNIS + 7 score; this treatment has the potential to halt progression of neuropathy (Citation105). Of ongoing recruiting trials, none specify the acceptance of patients with late-stage (FAP stage > II) ATTR amyloidosis with polyneuropathy (clinicaltrials.gov, accessed on 15 May 2015). eThere are limited published data for diflunisal treatment of patients with FAP stage III (4 patients with PND stage IV received diflunisal in the Diflunisal Trial (Citation52)). fLiver transplant is proposed according to eligible criteria (health status, no evolutive cancer, compliance in the anti-rejection treatment). Best outcome recorded for early-onset (≤ 50 years of age) Val30Met patients. ATTR = transthyretin amyloidosis; FAP = familial amyloidosis with polyneuropathy; PND = polyneuropathy disability; TTR = transthyretin.