Figures & data
Figure 1. Hypoxia-inducible factor (HIF) regulation.
Notes: Under normoxic conditions, HIF-α is hydroxylated in the oxygen-dependent destruction domain (Pro402 or 564 of HIF-1 α) and at an asparagine residue in the C-terminal transactivation domain (Asn803 of HIF-1 α). Prolyl hydroxylation by prolyl hydroxylase domain (PHD) enzyme leads to binding of HIF-1 α to the pVHL–E3–ubiquitin ligase complex. HIF-α is consequently polyubiquitinated and degraded by the proteasome. Acetylation of HIF-α by arrest-defective-1 (ARD1) enhances binding to pVHL and subsequent ubiquitination. During hypoxia, prolyl hydroxylases are rendered inactive and HIF-α is stabilized and translocates to the nucleus, where it is SUMOylated by small ubiquitin-like modifier (SUMO) conjugases. Failure to deSUMOylate HIF-1 α [e.g., the absence of a sentrin/SUMO-specific protease 1 (SENP1)] targets HIF-1α for VHL/proteasome-dependent degradation by an alternate signal (prolyl hydroxylation independent) for pVHL binding. DeSUMOylated HIF-1 α escapes degradation, heterodimerizes with HIF-1β, and increases transcription of HIF target genes.
![Figure 1. Hypoxia-inducible factor (HIF) regulation.Notes: Under normoxic conditions, HIF-α is hydroxylated in the oxygen-dependent destruction domain (Pro402 or 564 of HIF-1 α) and at an asparagine residue in the C-terminal transactivation domain (Asn803 of HIF-1 α). Prolyl hydroxylation by prolyl hydroxylase domain (PHD) enzyme leads to binding of HIF-1 α to the pVHL–E3–ubiquitin ligase complex. HIF-α is consequently polyubiquitinated and degraded by the proteasome. Acetylation of HIF-α by arrest-defective-1 (ARD1) enhances binding to pVHL and subsequent ubiquitination. During hypoxia, prolyl hydroxylases are rendered inactive and HIF-α is stabilized and translocates to the nucleus, where it is SUMOylated by small ubiquitin-like modifier (SUMO) conjugases. Failure to deSUMOylate HIF-1 α [e.g., the absence of a sentrin/SUMO-specific protease 1 (SENP1)] targets HIF-1α for VHL/proteasome-dependent degradation by an alternate signal (prolyl hydroxylation independent) for pVHL binding. DeSUMOylated HIF-1 α escapes degradation, heterodimerizes with HIF-1β, and increases transcription of HIF target genes.](/cms/asset/457a982d-7740-443f-b13c-d98558d7b498/irnf_a_653754_f0001_b.jpg)
Figure 2. Regulation of hypoxia-inducible factor (HIF) prolyl hydroxylases.
Notes: HIF prolyl hydroxylase domains (PHDs) require oxygen, Fe2+, and 2-oxoglutarate for prolyl hydroxylation activity. Nitric oxide (NO), reactive oxygen species (ROS), the Krebs cycle metabolites succinate and fumarate, cobalt chloride (CoCl2), and Fe chelators such as desferroxamine may inhibit the PHD family of HIF prolyl hydroxylases during normoxia. A number of HIF prolyl hydroxylase inhibitors are being investigated for preclinical and clinical trials. NGF, nerve growth factor.
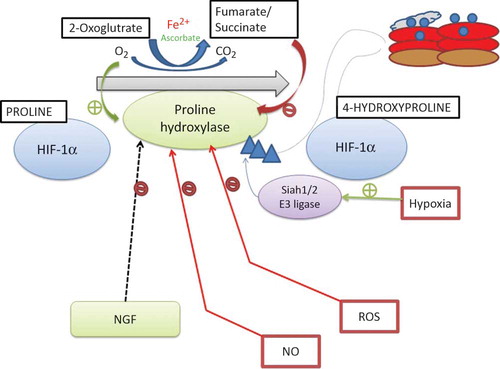
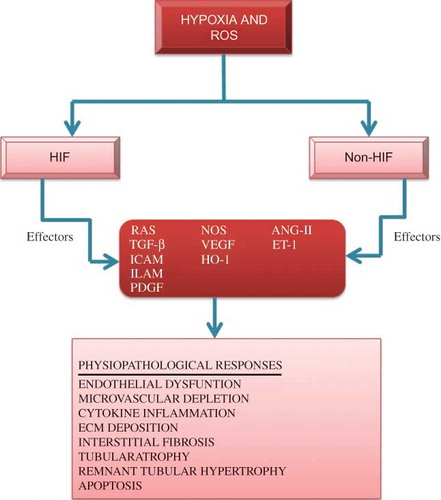
Figure 3. Representation for the association of hypoxia and reactive oxygen species (ROS) in hypoxia-driven renal parenchymal destructive processes and their related mediators.
Notes: HIF and non-HIF mechanisms, such as p53 or STAT3, are the key regulators of these processes. Some effectors may be protective as well as destructive and some with both potentially injurious and protective characteristics. ECM, extra cellular matrix; ICAM, intercellular adhesion molecule 1; ELAM, endothelial-leukocyte adhesion molecule; PDGF, platelet-derived growth factor; ET-1, endothelin-1; HO-1, hemeoxygenase-1; ANG-II, angiotensin-II; TGF-β, transforming growth factor-β; NOS, nitric oxide synthase; RAS, renin-angiotensin system.