Figures & data
Figure 1. Study design. We enrolled 35 patients in this study. These 35 patients with hemodialysis were randomly divided into two groups, a sarpogrelate group (n = 17) and a cilostazol group (n = 18). Antiplatelet agents other than aspirin were stopped for 4 weeks as a washout period. Only aspirin was continued if the patients had previously been treated with aspirin. Four weeks after the washout period, the patients were treated with sarpogrelate or cilostazol for 24 weeks. All 35 patients finished the study.
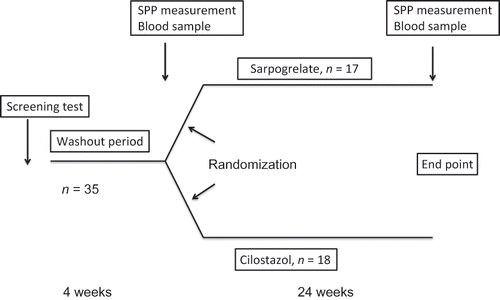
Table 1. Basic patient characteristics.
Figure 2. Changes in SPP. Data are expressed as mean ± SD.
Notes: Open squares indicate SPP levels at pretreatment and filled squares indicate mean SPP levels at 24 weeks. *p < 0.05, **p < 0.01 versus pretreatment values.
SPP levels significantly increased at 24 weeks from pretreatment levels in both groups.
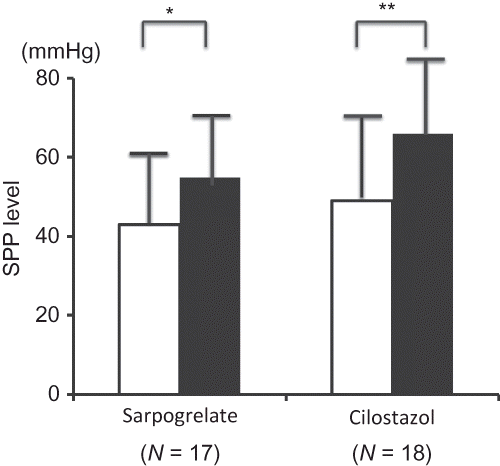
Table 2. Changes in serum and plasma parameters.
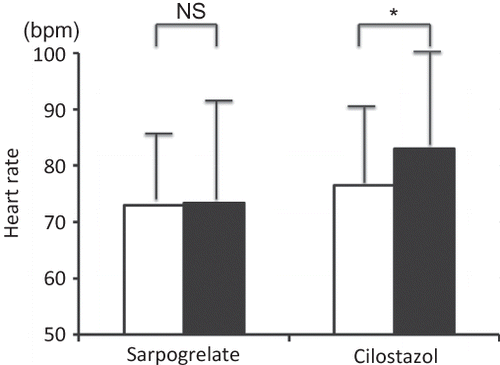
Figure 3. Changes in heart rate. Data are expressed as mean ± SD. Heart rate in the sarpogrelate group and in the cilostazol group.
Notes: Open squares indicate heart rates at pretreatment and filled squares indicate heart rates at 24 weeks.
*p < 0.05 versus pretreatment value; NS, not significant.
No significant differences were observed between the two groups at pretreatment and at 24 weeks. In the cilostazol group, heart rate significantly increased at 24 weeks.