Figures & data
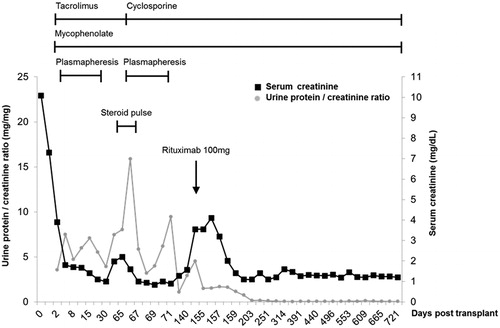
Figure 1. The first allograft biopsy on day 54 after transplantation showed no evidence of cellular or humoral rejection on light microscopy (A), however, revealed foot process effacement of podocytes on electron microscopy (B) which was compatible with focal segmental glomerulosclerosis.
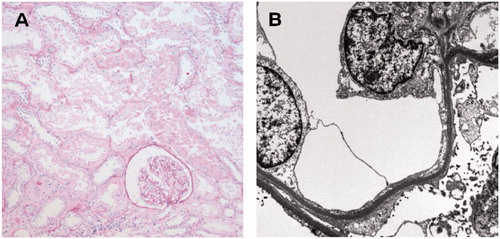
Figure 2. The second allograft biopsy on day 144 after transplantation showed a glomerulus with focal segmental sclerosis on light microscopy (A). Prominent foot process effacement of podocytes was also noted on electron microscopy (B).
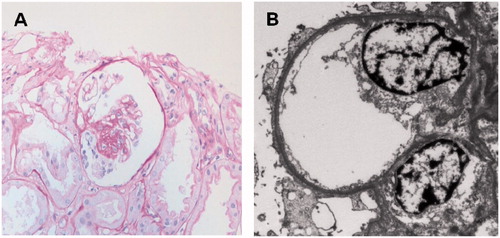
Figure 3. Clinical course in terms of serum creatinine (mg/dL, black line) and urinary protein-to-creatinine ratio (mg/g, gray line). After a single dose of rituximab 100 mg without further plasmapheresis, the patient maintained complete remission state of proteinuria with normal creatinine level for 18 months.