Figures & data
Table 1. Main characteristics of the studies included in this meta-analysis.
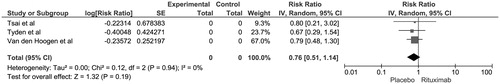
Figure 1. Forest plot of included RCTs comparing risk of biopsy-proven acute rejection in recipients with rituximab induction versus control; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error.
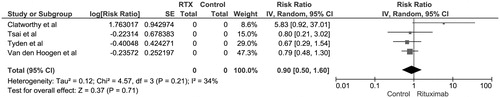
Figure 2. Forest plot of included RCTs comparing risk of biopsy-proven acute rejection in recipients with rituximab induction versus placebo; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error.