Figures & data
Figure 1. Fresh and aged plasmas trigger oxidative burst and protein phosphorylation in neutrophils. (A) Representative results of ROS generation in untreated normal human neutrophils (Control), and neutrophils treated for 1 h with fresh (1 day preparation) non-leukocyte-reduced plasma (NLR-1D), aged (42 day preparation) non-leukocyte-reduced plasma (NLR-42D), aged leukocyte-reduced plasma (LR-42D), and NLR-42D plus NADPH oxidase inhibitor DPI (NLR-42D + DPI). The results are expressed as means ± SD from 3 experiments. fMLP: formyl-Met-Leu-Phe (as a positive control). (*p < 0.05, compared with NLR-42D). (B) Western blot analysis of protein tyrosine phosphorylation in response to different preparations of plasma. Normal human neutrophils were incubated with the different plasma preparations for 1 h. Whole cell lysates were blotted with anti-pY20 antibody. (C) Normal human neutrophils were incubated with the different plasma preparations for 1 hr. Whole cell lysates prepared from these neutrophils were subjected to antibody array analysis with immunoblotted with anti-pY20-HRP. (D-E) Western blot analysis demonstrated tyrosine phosphorylation of IKK, p105 and p50 in response to plasma treatment (*p < 0.05, compared with NLR-42D). 1D represents the ratio of p-IKK value over IKK. 1E represents the ratio of p105 value over p50.
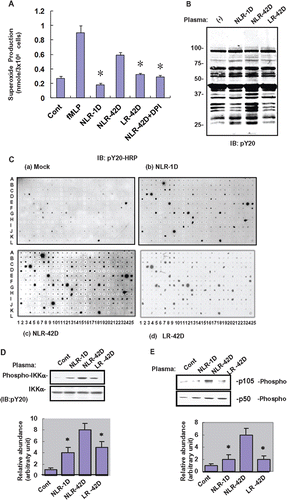
Figure 2. Fresh and aged plasmas induce nonmuscle type IIA myosin (MYH9) expression in neutrophils. (A) Of the immune cells tested, myosin type II was detected in T cells, B cells and macrophages, but not in neutrophils (B) The NLR-42D plasma group had significantly higher levels of MYH9 compared to the LR-42D plasma group (*p < 0.05). (C) NADPH oxidase inhibitor DPI abolished the plasma-induced expression of MYH9 in the NLR-42D group (*p < 0.05).
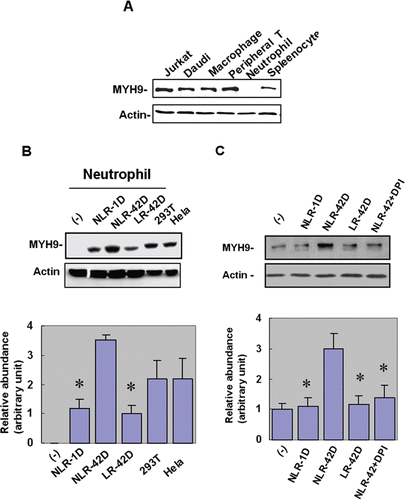
Figure 3. Blebbastatin, ascorbic acid, and STS disrupt migration-related morphological change in neutrophils. (A) Fresh and aged plasma induced neutrophil morphological changes (n = 100 cells). (B) Blebbistatin, ascorbic acid, and STS abrogated plasma-induced morphological changes in neutrophils (n = 7 blood samples from 7 different volunteers; in triplicate). DMSO was used as a vehicle control. (C) Aged plasma induced a greater number of morphological changes than fresh plasma (*p < 0.05, compared with NLR-42D) (n = 5 blood samples from 5 different volunteers; in triplicate) (D) Blebbistatin, ascorbic acid, and STS significantly abrogated plasma-induced neutrophil morphological change, (*p < 0.05, compared with NLR-42D).
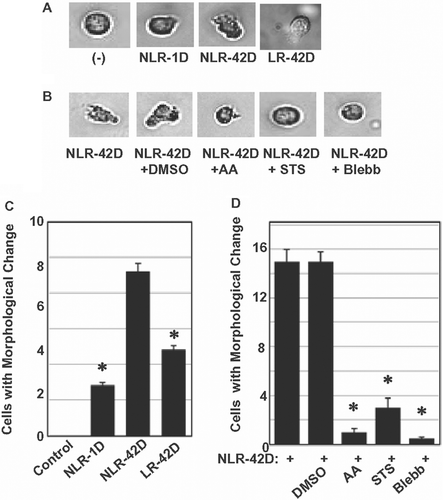
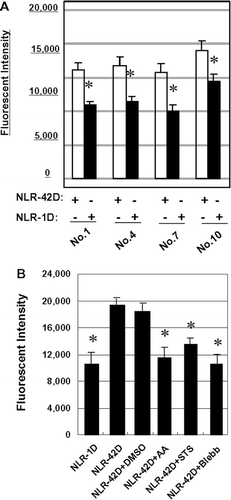
Figure 4. Blebbistatin, ascorbic acid, and STS suppress phagocytosis in plasma-treated neutrophils. Neutrophils were incubated with NLR-1D or NLR-42D from 4 different donors in triplicate (*p < 0.05, compared with NLR-42D). (B) Blebbistatin, ascorbic acid, and STS pretreatment significantly blocked plasmainduced phagocytosis in 6 different donors in triplicate (*p < 0.05, compared with NLR-42D).