Figures & data
Figure 1. Uptake of BODIPY fatty acid analogs into enterocytes. Mucosal explants were organ cultured in the presence of BODIPY 500/510 C1, C12 for the indicated periods of time. After culture and fixation, cryosections were labeled with antibodies to the brush border enzyme aminopeptidase N (ApN) as described in Methods. The arrows indicate the labeling of the brush border of four neighbouring enterocytes. In addition, the fatty acid analog mainly labeled the apical cytoplasm (C) of the enterocytes, whereas the enterocyte nuclei (N), goblet cells (G), and the underlying lamina propria (LP) were all devoid of labeling. Bars, 20 μm.
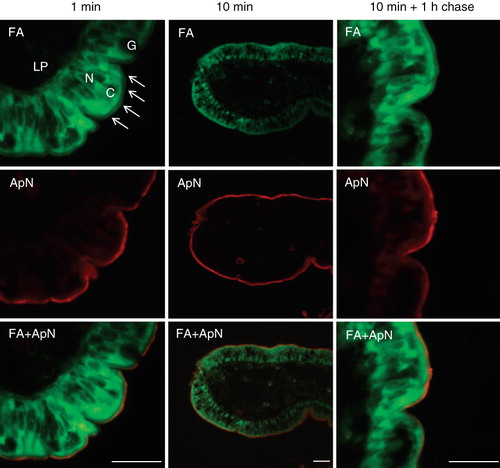
Figure 2. Localization of apolipoprotein B in enterocytes. Sections of mucosal explants, cultured in the presence of a BODIPY fatty acid analog for the indicated periods of time, were labeled with an antibody to apolipoprotein B. Labeling for this protein was mainly localized in intracellular punctae of the enterocytes, likely to represent lipoproteins. The fatty acid analog did not accumulate in the apolipoprotein B-positive punctae, neither by 10 min nor after 1 h of chase. Nuclei were visualized by DAPI staining in the merged images. Bars, 20 μm.
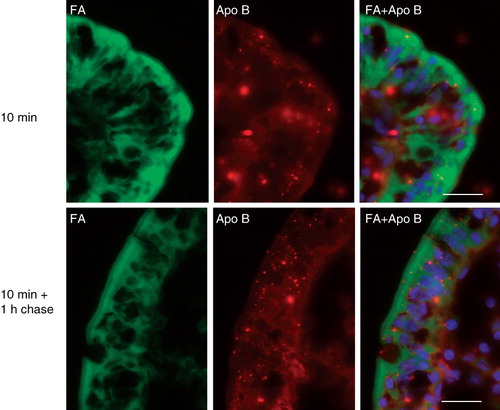
Figure 3. Rapid insertion of BODIPY fatty acids into the brush border. Fluorescent images of short-term labeled explants. Labeling of the enterocyte cytoplasm and brush border was evident after only few seconds of exposure to the analog. The fluorescence frequently appeared patch or punctate. Bars, 20 μm.
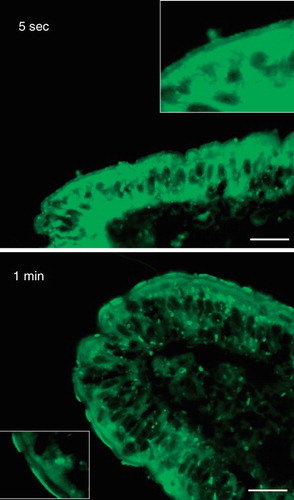
Figure 4. DRM analysis of lipid-treated microvillar membrane vesicles. Microvillar membrane vesicles were prepared, treated with a fat mixture, extracted with Triton X-100, and fractionated by sucrose gradient centrifugation as described in Methods. In the control, most of the protein in DRMs, including aminopeptidase N (ApN), alkaline phosphatase (AP), and annexin A2 (An A2) were mainly seen in the high-density floating fractions (4–8). The fat mixture treatment caused a specific redistribution of AP to the low-density floating fractions (9–12).
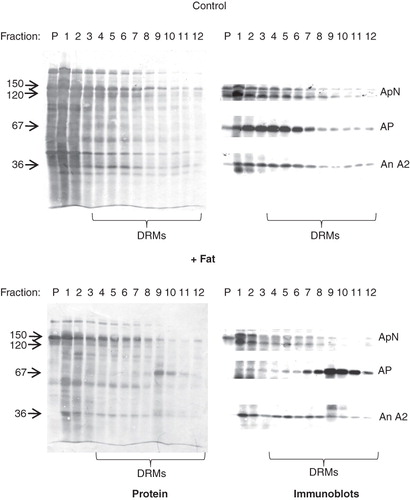
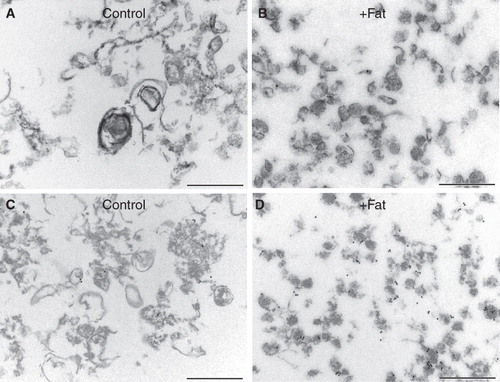
Figure 5. Morphology of microvillar low-density DRMs. Electron micrographs of low-density DRMs from control- and fat mixture-pretreated microvillar membranes. Whereas the former were relatively large and pleiomorphic, the latter were small and more uniformly vesicle-like. A markedly increased density of immunogold labeling for AP was seen after the fat treatment (C and D). Bars, 500 nm.