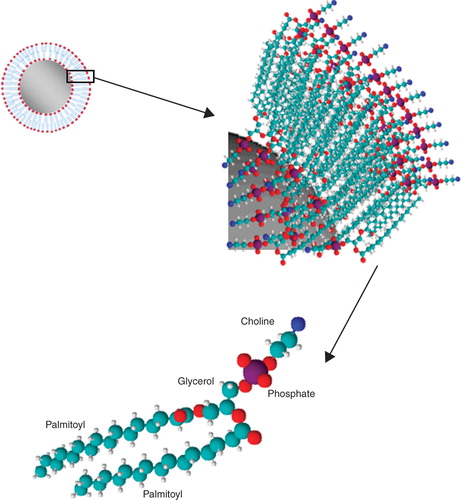
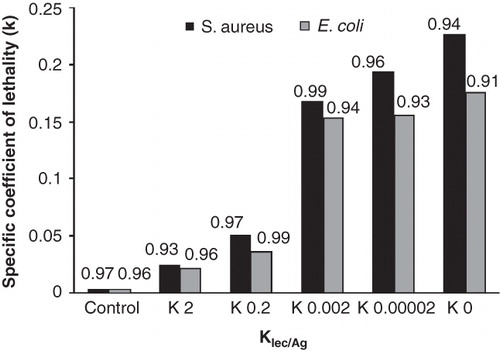
Figures & data
Figure 1. UV spectra of synthesized AgNPs solution (10 ppm) at two different lecithin concentration: (a) KLec/Ag = 1, and (b) KLec/Ag = 2. AgNPs are capped by lecithin molecules and lead to a reduction in their maximum absorption.
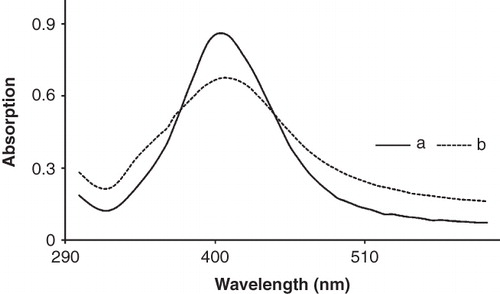
Figure 2. TEM images of prepared AgNPs with various lecithin concentrations: (a) Klec/Ag = 0; synthesized AgNPs have a short life without stabilizer and linked one to another and appear agglomerated silver particles; (b) Klec/Ag = 0.2, and (c) Klec/Ag = 2; lecithin molecules capped AgNPs and prevent them to link to one another.
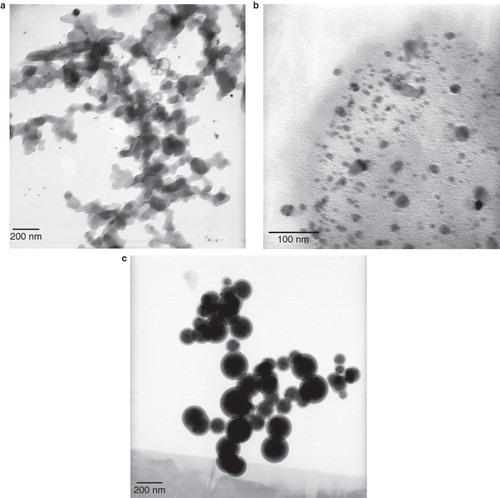
Figure 3. Size distribution histogram of AgNPs at two different lecithin concentrations: (a) KLec/Ag = 0.2, and (b) KLec/Ag = 2, increased lecithin concentration causes to increase size complex which indicates the presence of multilayer of lecithin molecules on AgNPs surface.
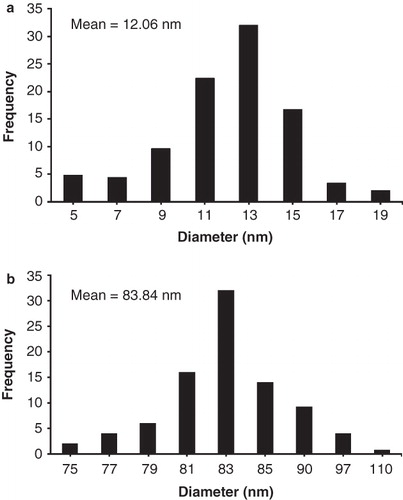
Table I. Mean value of MIC and MBC for synthesized AgNPs at phospholipid membrane with the ratio of KLec/Ag = 2 and determined by broth dilution method. Experiments were performed in duplicates.
Table II. Mean value of inhibition zone diameter of AgNPs with various amounts of lecithin. Experiments were performed in duplicates.
Figure 4. Photographic images of unstabilized AgNPs sedimentations (Klec/Ag = 0.002) in the CASO agar wells at various concentrations.
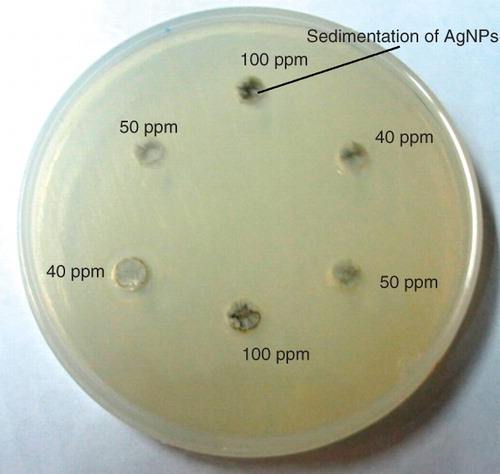
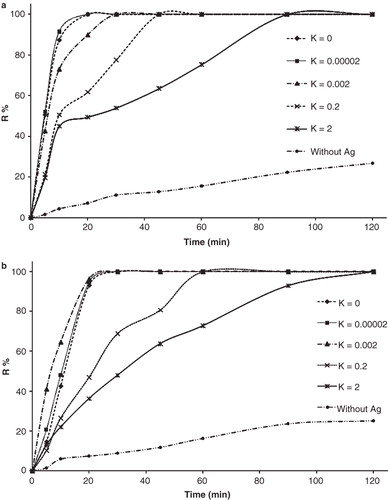
Figure 5. Bacterial inactivation Kinetics of (a) S. aureus and (b) E. coli which is calculated according to R% = (N0−N)/N0 × 100 during 120 min, in which N, and N0 are the number of CFU per milliliter of solution at the defined and initial time, respectively.